Gorgeous and mysterious electric eel. Electrochemical Proton Gradient An increase in the electrochemical gradient will lead to
Uncouplers, such as dinitrophenol, cause leakage of H across the membrane, greatly reducing the electrochemical proton gradient. Oligomycin specifically blocks the flow of protons through Rc
| Rice. 7-53. Changes in the redox potential during the passage of electrons during photosynthesis with the formation of NADPH and ATP) of plants and cyanobacteria. Photosystem II is very similar to the reaction center of purple bacteria (see Figure 7-50), with which it is evolutionarily related. Photosystem I differs from these two systems and is believed to be evolutionarily related to the photosystems of another group of prokaryotes - green bacteria. In photosystem I, excited chlorophyll electrons pass through a series of tightly bound iron-sulfur centers. Two series-connected photosystems provide a net flow of electrons from water to NADP to form NADPH. In addition, ATP is formed by ATP synthetase (not shown) due to the energy of the electrochemical proton gradient, which is created by the electron transport chain linking photosystem II to photosystem I. This Z-scheme of ATP formation is called non-cyclic phosphorylation in contrast to the cyclic scheme rice. 7-54 (see also fig. 7-52). |
When an uncoupling agent, such as dinitrophenol, is added to cells, oxygen uptake by the mitochondria is greatly increased as the rate of electron transfer increases. This acceleration is due to the existence of respiratory control. This control is believed to be based on the direct inhibitory effect of the electrochemical proton gradient on electron transport. When the electrochemical gradient disappears in the presence of an uncoupler, the uncontrolled electron transport reaches the maximum rate possible with a given amount of substrate. On the contrary, an increase in the proton gradient slows down the electron transport, and the process slows down. Moreover, if an unusually high electrochemical gradient is artificially created in the experiment on the inner membrane, normal electron transport will stop altogether, and in some parts of the respiratory chain it will be possible to detect a reverse flow of electrons. This last observation suggests that respiratory control simply reflects a balance between the values of free energy for pumping protons coupled with electron transport and for electron transport itself or, in other words, that the magnitude of the electrochemical proton gradient affects both the rate and the direction of electron transport in principle in the same way as the direction of action of ATP synthetase (Section 9.2.3).
The energy released in the process of electron transport along the respiratory chain is stored in the form of an electrochemical proton gradient on the inner membrane of mitochondria.
The pP (ArP) gradient causes P ions to move back into the matrix, and OP ions from the matrix, which enhances the effect of the membrane potential (AP), under the action of which any positive charge is attracted to the matrix, and any negative one is pushed out of it. The combined action of these two forces results in an electrochemical proton gradient (Fig. 7-19).
Almost all bacteria, including strict anaerobes, maintain a proton motive force on their membrane. The energy of the electrochemical proton gradient is used in them to rotate the bacterial flagellum, which allows the cell to move (Sec. 12.5.4), and for
The energy of the electrochemical proton gradient is used for ATP synthesis and transport of metabolites and inorganic ions into the matrix
On fig. 7-34 shows the levels of redox potential at different parts of the respiratory chain. A sharp drop takes place within each of the three major respiratory complexes. The potential difference between any two electron carriers is directly proportional to the energy released when an electron passes from one carrier to another (Fig. 7-34). Each complex acts as an energy-converting device, directing this free energy to move protons across the membrane, resulting in the creation of an electrochemical proton gradient as the electrons pass through the circuit. This energy conversion can be directly demonstrated by incorporating any isolated respiratory chain complex individually into liposomes (see Figure 7-25). In the presence of a suitable electron donor and acceptor, such a complex will transfer electrons, resulting in the pumping of protons across the liposome membrane.
Respiratory enzyme complexes couple the transport of electrons, accompanied by the release of energy, with the pumping of protons from the matrix. The electrochemical proton gradient created in this case delivers energy for ATP synthesis by another transmembrane protein complex, ATP synthetase, through which protons return to the matrix. ATP synthetase is a reversible coupling complex that normally converts the energy of the proton flux directed into the matrix into the energy of ATP phosphate bonds, but when the electrochemical proton gradient decreases, it is also able to use the energy of ATP hydrolysis to move protons out of the matrix. Chemiosmotic mechanisms are characteristic of both mitochondria and chloroplasts and bacteria, which indicates their exceptional importance for all cells.
As high-energy electrons pass through the respiratory chain, protons are pumped out of the matrix in each of its three energy-storing regions. As a result, an electrochemical proton gradient arises between the two sides of the inner membrane, under the influence of which protons return back to the matrix through ATP synthetase, a transmembrane enzyme complex that uses the energy of the proton current to synthesize ATP from ADP and P.
| Rice. 9-36. The proton motive force generated at the bacterial plasma membrane ensures the movement of nutrients into the cell and the removal of sodium to the outside. In the presence of oxygen (A), the respiratory chain of aerobic bacteria creates an electrochemical proton gradient, which is used by ATP synthetase to synthesize ATP. Under anaerobic conditions (B), the same bacteria obtain ATP as a result of glycolysis. Due to the hydrolysis of a part of this ATP under the action of ATP synthetase, a transmembrane proton-motive force arises, which carries out transport processes. (As described in the text, there are bacteria in which the electron transport chain pumps out protons and, under anaerobic conditions, the final electron acceptor in this case is not oxygen, but other molecules.) |
The electrochemical proton gradient creates a proton driving force, measured in millivolts (mV). Since a pP (ArH) gradient of 1 pH unit is equivalent to a membrane potential of about 60 mV, the proton motive force will be equal to A - 60 (ArH). In a typical cell, this force on the inner membrane of a respiring mitochondrion is about 220 mV and is the sum of a membrane potential of about 160 mV and a pH gradient. close to - ] pH unit.
But the synthesis of ATP is not the only process that occurs due to the energy of the electrochemical gradient. In the matrix, where the enzymes involved in the citric acid cycle and other metabolic reactions are located, it is necessary to maintain high concentrations of various substrates, in particular, ADP and phosphate are required for ATP synthetase. Therefore, a variety of charge-carrying substrates must be transported across the inner membrane. This is achieved by various carrier proteins embedded in the membrane (see section 6.4.4). many of which actively pump certain molecules against their electrochemical gradients, ie, carry out a process that requires the expenditure of energy. For most metabolites, the source of this energy is conjugation with the movement of some other molecules down their electrochemical gradient (see Section 6.4.9). For example, the ADP-ATP antiport system participates in ADP transport; when each ADP molecule enters the matrix, one ATP molecule leaves it along its electrochemical gradient. At the same time, the symport system couples the transition of phosphate into the mitochondria with the flow of P directed there, protons enter the matrix along their gradient and, at the same time, drag phosphate along with them. It is similarly transferred to the matrix and pyruvate (Fig. 7-21). The energy of the electrochemical proton gradient is also used to transfer Ca ions into the matrix, which, apparently, play an important role in the regulation of the activity of some mitochondrial enzymes, the absorption of these ions by mitochondria to remove them from the cytosol when the concentration of Ca in the latter becomes dangerous can also be of great importance. high (see section 12.3.7).
The action of ATP synthetase is reversible; it is able to use both the energy of ATP hydrolysis for pumping protons through the inner mitochondrial membrane, and the energy of proton flow along the electrochemical gradient for ATP synthesis (Fig. 7-26). Thus, ATP synthetase is a reversible conjugating system that carries out the interconversion of the energy of the electrochemical proton gradient and chemical bonds. The direction of its operation depends on the relationship between the steepness of the proton gradient and the local value of AG for ATP hydrolysis.
We have previously shown that the free energy of ATP hydrolysis depends on the concentration of three reactants - ATP, ADP and Pi (see Fig. 7-22). AG for ATP synthesis is the same value taken with a minus. The free energy of protons moving through the membrane is equal to the sum of (1) AG for moving one mole of any ions between areas with a difference in potentials AV and (2) AG for moving a mole of any molecules between areas with different concentrations. The equation for the proton motive force given in Sec. 7.1.7, combines the same components, but only the concentration difference is replaced by an equivalent increase in the membrane potential, so that an expression is obtained for the electrochemical potential of the proton. Thus, AG for the movement of protons and the proton-motive force take into account the same potential, only in the first case it is measured in kilocalories, and in the second - in millivolts. The coefficient for converting from one unit to another is the Faraday number. Thus, AGh = -0.023 (proton motive force), where AGh + is expressed in kilocalories per 1 mol (kcal/mol), and proton motive force is expressed in millivolts (mV). If the electrochemical proton gradient is 220 mV, then AGh = 5.06
If ATP synthetase does not normally transport P from the matrix, then the respiratory chain, located in the inner mitochondrial membrane, under normal conditions, transports protons through this membrane, thus creating an electrochemical proton gradient that delivers energy for ATP synthesis. Under certain conditions, it is possible to experimentally demonstrate the ability of the respiratory chain to pump protons from the matrix. It is possible, for example, to provide a suspension of isolated mitochondria with a suitable substrate for oxidation, and to block the flow of protons through ATP synthetase In such a flash of respiration, a sudden acidification of the medium can be detected using a sensitive rP electrode as a result of expulsion of P ions from the mitochondrial matrix.
| Rice. 7-36. Transfer of protons through the inner mitochondrial membrane with the participation of the uncoupling agent 2,4-dinitrophenol (DNP) The charged (protonated) form of DNP can freely |
Teiko Abe
How does the loss of the electrochemical gradient lead to heat generation?
It is my understanding that the decoupling of proton flow and ATP synthase provides a bypass for protons between the outer and inner membrane of the mitochondria, so that protons do not have to pass through ATP synthase on their way to the matrix. I see how this results in the loss of the electrochemical gradient. But why is heat generated?
AliceD ♦
For the same reason as when the battery is short-circuited :) Same principle, same effect.
Answers
Satvik Pasani
ALiceD's comment is completely true. (Although in real-life cases, the short circuit is rarely absolute, as there is usually some finite resistance in the short circuit wire.)
You can understand this in two ways.
Intuitive that the decoupling provides a channel for the movement of hydrogen ions across the membrane in the direction of their electrochemical gradient without any work. Therefore, the energy it receives by crossing the potential difference can be considered converted into kinetic energy, that is, the hydrogen ions are accelerated by the potential difference, which causes them to pick up speed and therefore move at a higher speed than the average of the last compartment. This will lead to more collisions (and more energetic ones) with the surrounding molecules, which will also slightly increase their kinetic energy, which will eventually increase the average kinetic energy, a measure of which is called temperature. If it were bound, the hydrogen ions would not gain kinetic energy, since the energy they gain by crossing the potential difference would be used to work in the ATP synthase mechanism.
Strictly speaking , you can show this using chemical thermodynamics, which involves using Δ G "role="presentation" style="position: relative;"> ΔG Δ G "role="presentation" style="position: relative;"> Δ G "role="presentation" style="position: relative;">Δ Δ G "role="presentation" style="position: relative;">g functions, μ "role="presentation" style="position: relative;"> μ μ "role="presentation" style="position: relative;"> μ "role="presentation" style="position: relative;">μ functions and some related thermodynamic variables. Let me know if you want this explanation (although I risk losing touch with the mathematical aspect of thermodynamics)
PS:- Although the thermodynamic explanation also takes into account the increase in temperature due to the erased concentration gradient, it is difficult to explain using the previous model. You can think of this as neutralizing the concentration gradient changes the number of collisions per unit volume (and time), and therefore also contributes to the observed temperature change.
WYSIWYG ♦
The way you explained the mechanism is very good +1
Teiko Abe
Thanks for your reply. Now I'm quite happy with the intuitive approach. I'm not sure I can fully understand the rigorous math explanation at this point, I feel like I need to do some reading first.
Electrochemical gradient, or electrochemical potential gradient- a set of concentration gradient and membrane potential, which determines the direction of movement of ions through the membrane. It consists of two components: a chemical gradient (concentration gradient), or the difference in concentrations of a solute on both sides of the membrane, and an electrical gradient (membrane potential), or the difference in charges located on opposite sides of the membrane. The gradient arises due to the unequal concentration of ions on opposite sides of the permeable membrane. Ions move across the membrane from an area of higher concentration to an area of lower concentration by simple diffusion. Ions also carry an electric charge, which forms an electric potential on the membrane (membrane potential). If there is an uneven distribution of charges on both sides of the membrane, then the difference in electrical potential generates a force that results in ionic diffusion until the charges on both sides are balanced.
Encyclopedic YouTube
1 / 3
✪ Membrane potentials - Part 1
✪ In Da Club - Membranes & Transport: Crash Course Biology #5
✪ Neuronal synapses (chemical) | Human anatomy and physiology | Health & Medicine | Khan Academy
Subtitles
I'll draw a small cage. It will be a typical cell, and it is filled with potassium. We know that cells love to accumulate it inside themselves. Lots of potassium. Let its concentration be somewhere around 150 millimoles per liter. Huge amount of potassium. Let's put it in parentheses, because parentheses denote concentration. There is also some potassium on the outside. Here the concentration will be approximately 5 millimoles per liter. I will show you how the concentration gradient will be set. It doesn't happen on its own. This requires a lot of energy. Two potassium ions are pumped in, and at the same time three sodium ions leave the cell. So potassium ions get inside initially. Now that they're inside, will they be held here on their own? Of course not. They find anions, small molecules or atoms with a negative charge, and position themselves near them. Thus, the total charge becomes neutral. Each cation has its own anion. And usually these anions are proteins, some structures that have a negative side chain. It can be chloride, or, for example, phosphate. Anything. Any of these anions will do. I'll draw a few more anions. So here are two potassium ions that just got inside the cell, this is what it looks like now. If everything is good and static, then this is how they look. And in fact, to be completely fair, there are also small anions here, which are here on a par with potassium ions. There are small holes in the cell through which potassium can flow out. Let's see what it will look like and how it will affect what happens here. So we have these little channels. Only potassium can pass through them. That is, these channels are very specific for potassium. Nothing else can pass through them. No anions, no proteins. Potassium ions, as it were, are looking for these channels and reasoning: “Wow, how interesting! So much potassium here! We should go outside." And all these potassium ions just leave the cell. They go outside. And as a result, an interesting thing happens. Most of them have moved outside. But there are already a few potassium ions outside. I said that there was this little ion here, and it could theoretically get inside. He can get into this cage if he wants to. But the fact is that in total, in total, you have more movements outward than inward. Now I'm erasing this path because I want you to remember that we have more potassium ions that tend to escape due to the presence of a concentration gradient. This is the first stage. Let me write it down. The concentration gradient causes potassium to move outward. Potassium begins to move out. Comes out of the cell. And then what? Let me draw it in the process of going outside. This potassium ion is here now, and this one is here. Only anions remain. They remained after the departure of potassium. And these anions begin to produce a negative charge. Very large negative charge. Only a few anions moving back and forth create a negative charge. And the potassium ions on the outside think it's all very interesting. There is a negative charge here. And since it is there, they are attracted to it, since they themselves have a positive charge. They are drawn to a negative charge. They want to return. Now think. You have a concentration gradient that pushes potassium out. But, on the other hand, there is membrane potential, - in this case negative - which occurs due to the fact that potassium left behind an anion. This potential stimulates potassium to come back. One force, concentration, pushes the potassium ion out, another force, the membrane potential, which is created by potassium, forces it back in. I'll free up some space. Now I will show you something interesting. Let's build two curves. I'll try not to miss anything on this slide. I'll draw everything here and then a small fragment of it will be visible. We build two curves. One of them will be for the concentration gradient, and the other for the membrane potential. It will be potassium ions outside. If you follow them for time - this time - you get something like this. Potassium ions tend to go outside and reach equilibrium at a certain point. Let's do the same with time on this axis. This is our membrane potential. We start at the zero time point and get a negative result. The negative charge will get bigger and bigger. We start at the zero point of the membrane potential, and it is at the point where potassium ions begin to come out that the following happens. V in general terms everything is very similar, but it happens as if in parallel with changes in the concentration gradient. And when these two values equalize with each other, when the number of potassium ions going out is equal to the number of potassium ions that come back in, you get such a plateau. And it turns out that the charge in this case is minus 92 millivolts. At this point, where there is practically no difference in terms of the total movement of potassium ions, equilibrium is observed. It even has its own name - "equilibrium potential for potassium." Upon reaching the value of minus 92 - and it differs depending on the type of ions - upon reaching minus 92 for potassium, an equilibrium of potentials is created. I will write down that the charge for potassium is minus 92. This happens only when the cell is permeable to only one element, for example, to potassium ions. And still the question may arise. You might be thinking, “Wait a second! If the potassium ions move outward - which they do - then don't we have a lower concentration at a certain point, because the potassium has already gone out of here, and a higher concentration here is provided by moving the potassium outward? Technically it is. Here, outside, contains more potassium ions. And I did not mention that the volume also changes. This results in a higher concentration. And the same is true for the cell. Technically, there is a lower concentration. But actually I didn't change the value. And the reason is the following. Look at these values, these are moths. And that's a huge number, right? 6.02 times 10 to the minus 23 power is not a small number at all. And if you multiply it by 5, it will come out approximately - let me quickly calculate what we got. 6 multiplied by 5 is 30. And here are millimoles. 10 to 20 moles. It's simple great amount potassium ions. And to create a negative charge, they need very little. That is, the changes caused by the movements of the ions will be insignificant compared to 10 to the 20th power. This is why concentration changes are not taken into account.
Overview
The electrochemical potential is used in electroanalytical chemistry, and in industry it is used in the manufacture of batteries and fuel cells. It is one of many interchangeable forms of potential energy in which energy can be conserved.
In biological processes, ions pass through the membrane by diffusion or active transport, determined by an electrochemical gradient. In mitochondria and chloroplasts, proton gradients are used to generate chemiosmotic potential, which is also known as proton- driving force ∆p or ∆μH+. This potential energy is used to synthesize ATP through or photophosphorylation. The proton-motive force according to Mitchell's chemiosmotic theory is the common product of the coupled processes of respiration and oxidative phosphorylation. It consists of two factors: chemical (or osmotic) - the difference in concentrations of H + ions in the mitochondrial matrix and intermembrane space, and electrical - due to the difference in electric charges located on opposite sides of the membrane. The difference in the concentrations of H + ions, measured in units of pH, is denoted ΔpH. The difference in electrical potentials is denoted by the symbol Δψ. Therefore, the equation takes the form:
Δ μ H + = Δ ψ + Δ p H (\displaystyle \Delta \mu _(H^(+))=\Delta \psi +\Delta pH) ,
Δ p H = p H A − p H B (\displaystyle \Delta pH=pH_(A)-pH_(B))
the difference in concentrations of H + ions (chemical gradient) on the A(+)-side and B(-)-side of the membrane.
The electrochemical gradient is similar to the pressure that water exerts as it flows through a hydroelectric dam. Membrane transport proteins, such as sodium-potassium ATPase, are analogous to turbines that convert the potential energy of water into other forms of physical or chemical energy, and the ions that pass through the membrane are analogous to water that falls to the bottom of a dam. In addition, the energy can be used to pump water to a lake upstream of the dam. Similarly, chemical energy in cells can be used to create electrochemical gradients.
Chemistry
The term "electrochemical potential" is usually used in cases where a chemical reaction must occur, for example, with the transfer of an electron in an electric battery. In batteries, the electrochemical potential arising from the movement of ions balances the reaction energy of the electrodes. The maximum voltage that a battery reaction can produce is called the standard electrochemical potential for that reaction. Along with macroergic compounds, chemical energy can be stored on biological membranes that function like capacitors, which act as an insulating layer for charged ions.
biological significance
Generation of a transmembrane electrical potential through the movement of ions through cell membrane results in biological processes such as nerve conduction, muscle contraction, hormone secretion, and sensory responses. It is believed that the membrane of a typical animal cell has a transmembrane electrical potential from -50 mV to -70 mV.
Electrochemical gradients also play a role in the establishment of proton gradients of oxidative phosphorylation in mitochondria. The final stage of cellular respiration is the electron transfer chain. Four built-in complexes in the inner membrane mitochondria (cristae) make up the electron transport chain. However, only complexes I, III, and IV are proton pumps and pump protons from the matrix into the intermembrane space. In total, ten protons are obtained, which move from the matrix to the intermembrane space, generating an electrochemical potential of more than 200 mV. This sets in motion the flow of protons back into the matrix through ATP synthase, which synthesizes ATP by attaching an inorganic phosphate to an ADP molecule. Thus, the generation of a proton electrochemical gradient is critical for energy synthesis in mitochondria. The general equation for the electron transport chain looks like this:
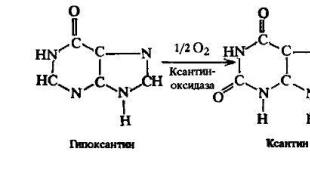
NADH + 11 H + (matrix) + 1 / 2 O 2 ⟶ NAD + + 10 H + (IMS) + H 2 O (\displaystyle NADH+11H^(+)(matrix)+1/2\ O_(2) \longrightarrow NAD^(+)+10H^(+)(IMS)+H_(2)O) .
The electron transport chain of photosynthesis in plants acts similarly to the respiratory chain of electron transport, where protons are pumped into the lumen of chloroplasts (the thylakoid lumen), and the resulting gradient is used to synthesize ATP through the enzyme ATP synthase. The proton gradient can be generated using non-cyclic or cyclic photophosphorylation. Proteins that are involved in non-cyclic photophosphorylation, photosystem II (PSII) and the cytochrome b6f complex are directly capable of generating a proton gradient. For every four photons absorbed by PSII, there are eight protons that are pumped into the lumen (the thylakoid lumen) from the stroma. The general equation for photophosphorylation is as follows:
2 H 2 O + 6 H + (stroma) + 2 NADP + ⟶ O 2 + 8 H + (lumen) + 2 NADPH (\displaystyle 2H_(2)O+6H^(+)(stroma)+2NADP^(+ )\longrightarrow O_(2)+8H^(+)(lumen)+2NADPH) .
Several other transporters and ion channels play a role in generating the proton electrochemical gradient. One of them is the TPK 3 -potassium ion channel activated by Ca 2+ ions. It moves K+ ions from the lumen to the stroma, which help establish a pH gradient (concentration gradient) within the stroma. On the other hand, the electrically neutral antiporter K + (KEA 3) transports K + ions into the lumen and H + into the stroma, maintaining the ion balance and not disturbing the electric field.
Ionic Gradient
Since ions carry a charge, they cannot pass through the membrane by facilitated diffusion. The transfer of ions across the membrane is possible in two ways, through active or passive transport. An example of active ion transport is the work of Na + -K + -ATPase. It catalyzes the reaction of ATP hydrolysis to ADP and inorganic phosphate Fn. Hydrolysis of one ATP molecule releases energy, which changes the conformation of the enzyme, so that three Na + ions are transported outside, and two K + ions are transported inside the cell. As a result, the contents of the cell become more negatively charged than environment, an electric potential (EMF) V m ≈ -60 mV is generated. An example of passive transport is the current of ions through ion channels (channels for Na + , K + , Ca 2+ and Cl -) along the concentration gradient, from the region of higher concentration to the region of lower. For example, since there is a high concentration of Na + outside the cell, Na + ions will tend to enter the cell through the sodium ion channel. Since the electrical potential inside the cell is negative, the influx of positive ions will cause the membrane to depolarize, resulting in a shift in the value of the transmembrane electrical potential closer to zero. However, Na + ions will continue to move down the concentration gradient as long as the driving force of the chemical gradient is greater than the electrical potential. After the effect of both gradients (chemical and electrical) balance each other (V m for Na + is about +70 mV), the influx of Na + ions will stop, since the driving force (ΔG) will become zero. The equation for the driving force is as follows:
Δ G = R T l n (C i n / C e x t) + Z F V m (\displaystyle \Delta G=RTln(C_(in)/C_(ext))+ZFV_(m)).
Proton gradients
Proton gradients are important as a form of energy storage in many various types cells. The gradient is commonly used to drive ATP synthase, flagellar rotation, or transport of metabolites across the membrane. This section will focus on three processes that help establish proton gradients in the respective cells: bacteriorhodopsin function, non-cyclic photophosphorylation, and oxidative phosphorylation.
Bacteriorhodopsin
Bacteriorhodopsin, found in archaea, forms the pathway for the proton gradient, via the proton pump. The operation of the proton pump relies on a proton carrier (rhodopsin) that moves from the side of the membrane with a low concentration of H + ions to the side with a higher concentration of H + . The proton pump of bacteriorhodopsin is activated by absorption of photons with a wavelength of 568 nm, this leads to photoisomerization of the Schiff base (SB) in retinal, causing its transition from trance- at 13- cis-shape. Photoisomerization is extremely fast and takes only 200 femtoseconds. As a result, rhodopsin undergoes a series of rapid conformational rearrangements: the Schiff base is displaced from the residues Asp85 and Asp212, causing the transfer of H+ ions to the remainder Asp85, and the state M1 (meta-I) is formed. The protein then transitions to the M2 (meta-II) state by separating the residue Glu204 from Glu194, which releases a proton into the environment. This state is relatively long-lived. The Schiff base is reprotonated over the residue Asp85, forming the state N. It is important that the second proton comes from Asp96, since its deprotonated state is unstable and is quickly reprotonated (re-protonated) by a proton from the cytoplasm. protonation Asp85 and Asp96 lead to repeated isomerization of SB, thus forming the state O. Also, the residue Asp85 releases its proton Glu204 and bacteriorhodopsin returns to its resting state.
Photophosphorylation
Upon release from PSII, the reduced plastoquinone PQH 2 translocates to the cytochrome b6f complex, which transfers two electrons from PQH 2 to the plastocyanin y protein in two separate reactions. This process is similar to the Q-cycle occurring in ETC complex III. In the first reaction, plastoquinol PQH 2 binds to the complex from the lumen side and one electron passes to the iron-sulfur center (Fe-S), which then transfers it to cytochrome f, the latter transfers an electron to the plastocyanin molecule. The second electron passes to the heme molecule b L , which then transfers it to the heme b H , the latter transfers the electron to the second plastoquinone molecule PQ,. In the second reaction, the 2nd plastoquinol molecule PQH 2 is oxidized, donating an electron to another plastocyanin molecule and a half-reduced PQ, which is reduced to PQH 2 and leaves the complex. Both reactions are accompanied by the transfer of four protons per lumen.
Oxidative phosphorylation
NADH + H + + UQ + 4 H + (matrix) ⟶ NAD + + UQH 2 + 4 H + (IMS) (\displaystyle NADH+H^(+)+UQ+4H^(+)(matrix)\longrightarrow NAD ^(+)+UQH_(2)+4H^(+)(IMS))
Notes
- Nelson, David. Lehninger Principles of Biochemistry / David Nelson, Michael Cox. - New York: W.H. Freeman, 2013. - P. 403. - ISBN 978-1-4292-3414-6 .
- Nath, Sunil; Villadsen, John (2015-03-01). “Oxidative phosphorylation revisited” . Biotechnology and Bioengineering [English]. 112 (3): 429-437. DOI:10.1002/bit.25492. ISSN 1097-0290.
- Kolman J., Rem K.-G. Visual biochemistry. - M.: Mir, 2011. - S. 128-129. - 469 p. - 7000 copies. - ISBN 5-03-003304-1.
- Stroev E.A. Biological chemistry. - M. : Higher School, 1986. - S. 210. - 479 p.
- Yang, Huanghe; Zhang, Guohui; Cui, Jianmin (2015-01-01). “BK channels: multiple sensors, one activation gate” . Membrane Physiology and Membrane Biophysics. 6 : 29. DOI: 10.3389/fphys.2015.00029 . PMC. PMID.
- Shattock, Michael J.; Ottolia, Michela; Bers, Donald M.; Blaustein, Mordecai P.; Boguslavskyi, Andrii; Bossuyt, Julie; Bridge, John H. B.; Chen-Izu, Ye; Clancy, Colleen E. (2015-03-15). “Na+/Ca2+ exchange and Na+/K+-ATPase in the heart” . The Journal of Physiology [English]. 593 (6): 1361-1382. DOI:10.1113/jphysiol.2014.282319 . ISSN 1469-7793. PMC. PMID.
- Aperia, Anita; Akkuratov, Evgeny E.; Fontana, Jacopo Maria; Brismar, Hjalmar (2016-04-01). “Na+-K+-ATPase, a new class of plasma membrane receptors” . American Journal of Physiology - Cell Physiology [English]. 310 (7): C491-C495. DOI:10.1152/ajpcell.00359.2015 . ISSN 0363-6143. PMID.
- Nelson, David. Lehninger Principles of Biochemistry / David Nelson, Michael Cox. - New York: W.H. Freeman, 2013. - P. 464. - ISBN 978-1-4292-3414-6 .
- Poburko, Damon; Demaurex, Nicolas (2012-04-24). “Regulation of the mitochondrial proton gradient by cytosolic Ca2+ signals” . Pflugers Archive - European Journal of Physiology [English]. 464 (1): 19-26.
electrochemical gradient The ion is the driving force behind the flow of ions, which is a combination of the membrane potential (electrical gradient) and the concentration gradient of substances (chemical gradient). The electrical gradient characterizes the movement of only ions and is directed towards their opposite charge. The chemical gradient is directed from an area of high solute concentration to an area of low concentration.
The transport of substances across the membrane can occur passively and actively. active transport requires energy and passive carried out without energy consumption. Active transport always goes against the electrochemical gradient. Passive transport of solutes can only occur along a favorable electrochemical gradient.
Solute transport systems can be classified based on the use cellular energy.

1. Passive transport does not require ATP hydrolysis and is not associated with the transfer of another solute.
Diffusion of fat soluble substances (eg O 2 , CO 2 , alcohols and esters) can occur directly across the plasma membrane.
The transport of ions and small molecules often occurs through transmembrane proteins that serve ion channels(for various ions) or aquapores(for water molecules).
Ion channels have the following common components:
1)pore area, through which the ions diffuse.
2)selective filter inside the pore, whereby the channel is highly selective for certain ions (eg Na + channels).
3) canal gate, which open and close the channel. In the closed state, ions do not pass through the channel, but the channel is available for activation. In the open state, the ions move according to their electrochemical gradient. Channel gates can be controlled by one of the following mechanisms: membrane voltages (voltage dependent channels); chemical substances (chemodependent channels); mechanical forces in the membrane (stretch-dependent channels).
Diffusion can take place through carrier proteins called uniport, which selectively bind one solute to one side of the membrane and undergo a conformational change to deliver it to the other side. The transport of solutes through a uniport is called facilitated diffusion, because it's faster than simple diffusion. This is how glucose and amino acids are transported.

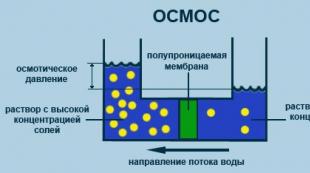
Osmosis - it is the movement (diffusion) of water through the aqua pores of the membrane, which is driven by a water concentration gradient. The water concentration is expressed in terms of the total solute concentration; the more dilute the solution, the lower the concentration of its solute and the higher the concentration of water. When two solutions are separated semipermeable membrane(which allows the transport of water but not solutes), water moves from a more dilute solution to a more concentrated one. Osmolarity is an expression of the osmotic force of the solution . Two solutions of the same osmolarity are called isosmotic. Solutions with greater than the osmolarity of the reference solution are called hyperosmotic, and solutions with lower osmolarity are called as hypoosmotic. An isotonic solution has the same osmolarity as functioning cells and does not cause pure water to move across their membrane; a hypotonic solution has a lower osmolarity than a functioning cell and causes the cells to swell, a hypertonic solution has a higher osmolarity than a cell and causes the cells to shrink. For example, if a patient is given intravenous hypotonic solution, the tone of the extracellular fluid initially decreases, and water moves into the intracellular fluid by osmosis (cells swell). Conversely, if you enter hypertonic solution, the tone of the extracellular fluid is increased, and water leaves the intracellular fluid (cells shrivel).
While the artificial lipid membrane is practically impermeable to ions, biological membranes contain " ion channels ”, through which individual ions selectively penetrate the membrane (see). The permeability and polarity of the membrane depend on electrochemical gradient, that is, on the concentrations of ions on both sides of the membrane ( concentration gradient) and from differences electrical potentials between the inner and outer sides of the membrane ( membrane potential).
In the resting state of cells, the membrane potential ( resting potential, see) ranges from −0.05 to −0.09 V, that is, an excess of negative charges predominates on the inner side of the plasma membrane. The rest potential is provided primarily by Na + and K + cations, as well as organic anions and the Cl - ion (1). The concentrations outside and inside the cell and the permeability coefficients of these ions are shown in Table (2).
The distribution of ions between the external environment and the internal volume of the cell is described Nernst equation(3), where ΔΨ G is the transmembrane potential (in volts, V), that is, the difference in electrical potentials between the two sides of the membrane in the absence of ion transport through the membrane ( equilibrium potential). For monovalent ions at 25°C, the factor RT/Fn is 0.026 V. At the same time, it follows from table (2) that for K + ΔΨ G ions is approximately −0.09 V, i.e., a value of the same order as and resting potential. For Na + ions, on the contrary, ΔΨ G ≈ +0.07 V, that is, higher than the resting potential. Therefore, Na + ions enter the cell when the Na + channel is opened. The inequality of the concentrations of Na + and K + ions is constantly maintained Na + /K + -ATPase when spending ATP (see).
Articles of the section "Conservation of energy on membranes":
- A. Electrochemical Gradient
2012-2019. Visual biochemistry. Molecular biology. Vitamins and their functions.
The reference book in a visual form - in the form of color schemes - describes all biochemical processes. Biochemically important chemical compounds, their structure and properties, the main processes with their participation, as well as the mechanisms and biochemistry of the most important processes in living nature are considered. For students and teachers of chemical, biological and medical universities, biochemists, biologists, physicians, as well as all those interested in life processes.