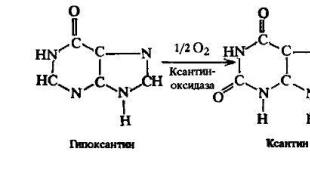
Cytoskeleton. Microvilli. Cell wall. Specialized organelles and cell structures Microvilli of an animal cell
And in the collar-flagellar cells of sponges and other multicellular animals. In the human body, microvilli have epithelial cells of the small intestine, on which microvilli form a brush border, as well as mechanoreceptors of the inner ear - hair cells.
Microvilli are often confused with cilia, but they differ dramatically in structure and function. The cilia have a basal body and a cytoskeleton of microtubules, are capable of rapid movements (except for modified immobile cilia) and serve in large multicellular organisms usually to create fluid currents or to perceive stimuli, and in unicellular and small multicellular animals also for movement. Microvilli do not contain microtubules and can only bend slowly (in the intestine) or are motionless.
For the ordering of the actin cytoskeleton of microvilli, auxiliary proteins that interact with actin - fimbrin, spectrin, villin, etc. - are responsible. Microvilli also contain cytoplasmic myosin of several varieties.
Intestinal microvilli (not to be confused with multicellular villi) greatly increase the surface area of absorption. In addition, in vertebrates, digestive enzymes are fixed on their plasmalemma, which provide parietal digestion.
The microvilli of the inner ear (stereocilia) are interesting in that they form rows with different, but strictly defined lengths in each row. The tops of the microvilli of the shorter row are connected to the longer microvilli of the neighboring row with the help of proteins - protocadherins. Their absence or destruction can lead to deafness, since they are necessary for opening sodium channels on the membrane of hair cells and, therefore, for converting mechanical sound energy into a nerve impulse.
Although microvilli persist on hair cells throughout life, each of them is constantly renewed by treadmilling of actin filaments.
Write a review on the article "Microvillus"
Links
Notes (edit)
Excerpt from Microvillus
It was already late in the evening when they entered the Olmüts Palace, occupied by the emperors and their entourage.On this very day there was a council of war, which was attended by all members of the gofkriegsrat and both emperors. At the council, contrary to the opinion of the old people - Kutuzov and Prince Schwarzernberg, it was decided to immediately attack and give a general battle to Bonaparte. The council of war had just ended when Prince Andrey, accompanied by Boris, came to the palace to look for Prince Dolgorukov. All the faces of the main apartment were still under the charm of today's military council, victorious for the party of young people. The voices of the procrastinators, advising to expect something else without coming, were so unanimously drowned out and their arguments were refuted by undoubted evidence of the benefits of the offensive, that what was being interpreted in the council, the future battle and, no doubt, victory, seemed no longer the future, but the past. All the benefits were on our side. Huge forces, no doubt superior to those of Napoleon, were concentrated in one place; the troops were animated by the presence of the emperors and rushed to work; the strategic point at which it was necessary to act was known to the smallest details of the Austrian general Weyrother, who was in charge of the troops (as if by a happy coincidence, the Austrian troops were on maneuvers last year on those fields on which they now had to fight the French); up to the smallest details, the surrounding area was known and conveyed on the maps, and Bonaparte, apparently weakened, did nothing.
Dolgorukov, one of the most ardent supporters of the offensive, has just returned from the council, tired, exhausted, but animated and proud of his victory. Prince Andrey introduced the officer he was patronizing, but Prince Dolgorukov, courteously and firmly shaking his hand, said nothing to Boris and, obviously unable to refrain from expressing those thoughts that most of all occupied him at that moment, he turned to Prince Andrey in French.
- Well, my dear, what a battle we have withstood! God grant only that that which will be a consequence of it, would be just as victorious. However, my dear, ”he said fragmentarily and animatedly,“ I must admit my guilt before the Austrians, and especially before Weyrother. What a precision, what a detail, what a knowledge of the area, what a foresight of all possibilities, all conditions, all the smallest details! No, my dear, it is impossible to deliberately invent anything more advantageous than the conditions in which we find ourselves. Combining Austrian clarity with Russian courage - what more do you want?
- So the offensive is finally decided? - said Bolkonsky.
For the normal functioning of the human body, food intake is needed. The absorption of substances and products of their breakdown necessary for life is carried out precisely in the small intestine. The intestinal villi located in it carry out this function. Their anatomy, placement, cytology will be discussed further.
The structure of the small intestine, its functions
In human anatomy, there are 3 sections - duodenal, skinny and iliac. The first is about 30 cm long. Special enzymes from the intestinal epithelium, bile and pancreatic enzymes come here. The suction process begins in the same section. Water and salts, amino acids and vitamins, fatty acids are actively absorbed by the villi.
There is no clear external boundary between the skinny and the iliac, and the total length is 4.5-5.5 m.But internal differences, of course, do exist. :
- has a large wall thickness;
- her intestinal villi are longer and smaller in diameter, and their number is greater;
- it is better supplied with blood.
Still, the main function of the duodenum is the digestion of food. This process is carried out not only in the intestinal cavity, but also near the walls (parietal digestion), as well as inside cells (intracellular).
For the implementation of the latter, there are special transport systems in the mucous membrane, different for each ingredient. An additional function of this is suction. In the rest, this is the main function.
Villus placement and anatomy
Intestinal villi in the alimentary canal are located in all three sections of the small intestine and give them a velvety appearance. The length of each of the villi is approximately 1 mm and the placement is very dense. They are formed from protrusions of the mucous membrane. On one square millimeter of the surface of the first and second sections of the small intestine, there can be from 22 to 40 pieces, on the ileum - up to 30.

Outside, all intestinal villi are covered with epithelium. Each of the cells has many outgrowths called microvilli. Their number can reach 4 thousand per epithelial cell, which significantly increases the surface of the epithelium, and, as a consequence, the absorbing surface of the intestine.

All intestinal villi in the human alimentary canal have an axial origin at the apex of the villi and many blood capillaries located in the stroma.
Cellular composition of villi
It is the presence of a certain type of cells that is responsible for how the intestinal villi functions. But first things first:
Each villi, regardless of location, is lined with a layer of epithelium, consisting of 3 cell types: columnar epithelial cell, goblet exocrinocyte and endocrinocyte.
Enterocytes
This is the most common cell type in the villus epithelium. Its second name is columnar epithelial cells. Cells are prismatic. And the main function of the intestinal villi is performed by them. Enterocytes provide the movement from the gastrointestinal tract into the blood and lymph of the substances necessary for the body, which are supplied during eating.

Epithelial cells on the surface have a special border formed by microvilli. There are 60 to 90 of these microvilli per 1 μm 2. They increase the suction surface of each cell by 30-40 times. The glycocalyx located on the surface of the microvilli produces degrading enzymes.
One of the varieties of epithelial cells are cells with microfolds or the so-called M-cells. Their location is the surface of lymphatic follicles, both group and single. They are distinguished by a more flattened shape and a small number of microvilli. But at the same time, the surface is covered with micro-folds, with the help of which the cell is able to capture macromolecules and the intestinal lumen.
Goblet exocrinocytes and endocrinocytes
Single cells, the number of which increases from the duodenum to the iliac. These are typical mucous cells that accumulate and then release their secretions onto the surface of the mucous membrane. It is mucus that promotes the movement of food along the intestines and at the same time participates in the process of parietal digestion.
The appearance of the cell depends on the degree of accumulation of secretions in it, and the very formation of mucus occurs in the area of the Golgi apparatus. An empty cell that has completely secreted its secret is narrow and with a reduced nucleus.

It is endocrinocytes that synthesize and secrete biologically active substances that not only play a digestive function, but also play an important role in overall metabolism. The main location of these cells is the duodenum.
Functions
From the structure it becomes immediately clear what function the intestinal villi perform in the digestive process, therefore we will only briefly list them:
- Absorption of carbohydrates, proteins, amino acids, as well as their decomposition products. They are transmitted through the villi into the capillaries and, together with the blood, are transported to the portal system of the liver.
- Absorption of lipids, or rather chylomicrons, particles derived from lipids. They are transmitted by the villi to the lymphatic system and further to the circulatory system, bypassing the liver.
- Another function of the intestinal villi is secretory, secreting mucus for easier movement of food through the intestines.
- Endocrine, because some cells of the villi produce histamine and serotonin, secretin and many other hormones and biologically active substances.
Embryo initiation and regeneration after damage
We figured out what cells the intestinal villus consists of and how does it function, but when is it laid in the human body and from which cells? Let's figure this out.
At the end of the second month or the beginning of the third intrauterine development of a person, parts of the small intestine and its functional components - folds, villi, crypts begin to form from the intestinal endoderm.
Initially, epithelial cells do not have strict differentiation, only by the end of the third month they divide. Glycocalyx, on the microvilli that cover the epithelial cells, is laid in the fourth month of the baby's development.
In the fifth week, with the correct course of pregnancy, the serous membrane of the intestine is laid, and in the eighth - the muscular and connective tissue membrane of the intestine. All membranes are laid from the mesoderm (visceral layer) and connective tissue mesenchyme.

Although all cells and tissues are still laid in intrauterine development, the intestinal villi can be damaged during the performance of their functions. How is the restoration of areas where cells have died? By mitotic division of healthy cells located nearby. They simply take the place of their dead brothers and begin to fulfill their function.
Microvilli (microvilli) up to 1-2 microns in length and up to 0.1 microns in diameter are finger-like outgrowths covered with cytolemma. In the center of the microvillus there are bundles of parallel actin filaments attached to the cytolemma at the apex of the microvillus and on its sides. Microvilli increase the free cell surface. In leukocytes and connective tissue cells, the microvilli are short, in the intestinal epithelium they are long, and there are so many of them that they form the so-called brush border. Microvilli are mobile due to actin filaments.
Cilia and flagella are also mobile, their movements are pendulum-like, wavy. The free surface of the ciliated epithelium of the respiratory tract, vas deferens, fallopian tubes is covered with cilia up to 5-15 microns in length and 0.15-0.25 microns in diameter. At the center of each cilium is an axial filament (axoneme) formed by nine interconnected peripheral double microtubules that surround the axoneme. The initial (proximal) part of the microtubule ends in the form of a basal body located in the cytoplasm of the cell and also consisting of microtubules. In their structure, flagella are similar to cilia; they perform coordinated oscillatory movements due to the sliding of microtubules relative to each other.
Intercellular connections are formed at the points of contact of cells with each other, they provide intercellular interactions. Such connections (contacts) are divided into simple, serrated and tight. A simple connection is the convergence of cytolemmas of neighboring cells (intercellular space) at a distance of 15-20 nm. With a serrated connection, the protrusions (teeth) of the cytolemma of one cell enter (wedge) between the teeth of another cell. If the protrusions of the cytolemma are long, deeply embedded between the same protrusions of another cell, then such connections are called finger-like (interdigitation).
In special dense intercellular junctions, the cytolemma of neighboring cells is so close together that they merge with each other. This creates a so-called blocking zone, impermeable to molecules. If a tight connection of the cytolemma occurs in a limited area, then an adhesion spot (desmosome) is formed. The desmosome is a high electron density area with a diameter of up to 1.5 microns, which acts as a mechanical connection between one cell and another. Such contacts are more common between epithelial cells.
There are also gap-like joints (nexuses), the length of which reaches 2-3 microns. Cytolemmas in such compounds are spaced 2-3 nm apart. Ions and molecules easily pass through such contacts. Therefore, nexuses are also called conductive connections. So, for example, in the myocardium through the nexus excitation is transmitted from some cardiomyocytes to others.
Cilia and flagella
Cilia and flagella - organelles of special significance, participating in the processes of movement, are outgrowths of the cytoplasm, the basis of which is a karts of microtubules, called the axial thread, or axoneme (from the Greek. axis - axis and nema - thread). The length of cilia is 2-10 microns, and their number on the surface of one ciliated cell can reach several hundred. The only type of human cells with a flagellum - the sperm - contains only one flagellum with a length of 50-70 microns. The axoneme is formed by 9 peripheral pairs of microtubules by one centrally located pair; such a structure is described by the formula (9 x 2) + 2 (Fig. 3-16). Inside each peripheral pair, due to the partial fusion of microtubules, one of them (A) is complete, the second (B) is incomplete (2-3 dimers are common with microtubule A).
The central pair of microtubules is surrounded by a central envelope, from which radial doublets diverge to the peripheral doublets. The peripheral doublets are connected to each other by nexin bridges, and from microtubule A to microtubule B of the neighboring doublet there are "handles" of the protein dynein (see Fig. 3- 16), which has ATPase activity.
The beating of the cilium and flagellum is caused by the sliding of adjacent doublets in the axoneme, which is mediated by the movement of the dynein arms. Mutations that cause changes in the proteins that make up the cilia and flagella lead to various dysfunctions of the corresponding cells. With Kartagener's syndrome (syndrome of motionless cilia), usually due to the absence of dynein pens; patients suffer from chronic diseases of the respiratory system (associated with a violation of the function of cleansing the surface of the respiratory epithelium) and infertility (due to immobility of sperm).
The basal body, similar in structure to the centriole, lies at the base of each cilium or flagellum. At the level of the apical end of the body, microtubule C of the triplet ends, and microtubules A and B continue into the corresponding microtubules of the axoneme of the cilium or flagellum. With the development of the cilia or flagellum, the basal body plays the role of a matrix on which the assembly of axoneme components occurs.
Microfilaments- thin protein filaments with a diameter of 5-7 nm, lying singly in the cytoplasm, in the form of septae or bundles. In skeletal muscle, thin microfilaments form ordered bundles, interacting with thicker myosin filaments.
The corticolon (terminal) network is a zone of thickening of microfilaments under the plasmolemma, which is characteristic of a large number of cells. In this network, microfilaments are intertwined and "sewn" to each other using special proteins, the most common of which is filamin. The cortical network prevents abrupt and sudden deformation of the cell under mechanical influences and ensures smooth changes in its shape by rearrangement, which is facilitated by actin-generating (transforming) enzymes.
The attachment of microfilaments to the plasmolemma is carried out due to their connection with its integral ("anchor") proteins (integrins) - directly or through a number of intermediate proteins talin, vinculin and α-actinin (see Fig. 10-9). In addition, actin microfilaments attach to transmembrane proteins in special areas of the plasmolemma, called adhesion junctions or focal contacts, which bind cells to each other or cells to components of the intercellular substance.
Actin, the main protein of microfilaments, is found in monomeric form (G-, or globular actin), which is capable of polymerizing into long chains in the presence of cAMP and Ca2 + (F-, or fibrillar actin). Typically, the actin molecule looks like two spirally twisted filaments (see Figures 10-9 and 13-5).
In microfilaments, actin interacts with a number of actin-binding proteins (up to several dozen species) that perform various functions. Some of them regulate the degree of actin polymerization, while others (for example, filamin in the cortical network or fimbrin and villin in the microvillus) promote the binding of individual microfilaments into systems. In non-muscle cells, actin accounts for about 5-10% of the protein content; only about half of it is organized into filaments. Microfilaments are more resistant to physical and chemical influences than microtubules.
Functions of microfilaments:
(1) ensuring contractility of muscle cells (when interacting with myosin);
(2) providing functions associated with the cortical layer of the cytoplasm and plasmolemma (exo- and endocytosis, pseudopod formation and cell migration);
(3) movement of organelles, transport vesicles and other structures within the cytoplasm due to interaction with some proteins (minimyosin) associated with the surface of these structures;
(4) ensuring a certain stiffness of the cell due to the presence of a cortical network, which prevents the action of deformations, but itself, rebuilding, contributes to changes in the cell form;
(5) formation of a contractile constriction during cytotomy, which completes cell division;
(6) formation of a base ("frame") of some organelles (microvilli, stereocilia);
(7) participation in the organization of the structure of intercellular junctions (encircling desmosomes).
Microvilli are finger-like outgrowths of the cytoplasm of a cell with a diameter of 0.1 µm and a length of 1 µm, the basis of which is formed by actin microfilaments. Microvilli provide a manifold increase in the surface area of the cell, on which substances are broken down and absorbed. On the apical surface of some cells that actively participate in these processes (in the epithelium of the small intestine and renal tubules) there are up to several thousand microvilli, which together form a brush border.
Rice. 3-17. Diagram of the ultrastructural organization of microvilli. AMP - actin microfilaments, AB - amorphous substance (apical part of the microvillus), F, V - fimbrin and villin (proteins that form crosslinks in the AMP bundle), mm - minimiosin molecules (attaching the AMP beam to the plasma membrane of the microvillus), TC - terminal network AMP, C - spectrin bridges (attach TS to the plasmolemma), MF - myosin filaments, IF - intermediate filaments, HA - glycocalyx.
The framework of each microvillus is formed by a bundle containing about 40 microfilaments lying along its long axis (Fig. 3-17). In the apical part of the microvillus, this bundle is fixed in an amorphous substance. Its rigidity is due to cross-links of fimbrin and villin proteins, from the inside, the bundle is attached to the plasmolemma of the microvillus by special protein bridges (minimiosin molecules. At the base of the microvillus, the bundle microfilaments are woven into the terminal network, among the elements of which there are myosin filaments. , determines the tone and configuration of the microvillus.
Stereocilia- modified long (branching in some cells) microvilli - are detected much less frequently than microvilli and, like the latter, contain a bundle of microfilaments.
⇐ Previous123
Read also:
Microfilaments, microtubules and intermediate filaments as the main components of the cytoskeleton.
Actin microfilaments - structure, function
Actin microfilaments are polymeric filamentous formations with a diameter of 6-7 nm, consisting of actin protein. These structures are highly dynamic: at the end of the microfilament facing the plasma membrane (plus-end), actin is polymerized from its monomers in the cytoplasm, while depolymerization occurs at the opposite (minus-end).
Microfilaments, thus, have a structural polarity: the growth of the filament comes from the plus-end, shortening - from the minus-end.
Organization and functioning actin cytoskeleton are provided by a number of actin-binding proteins that regulate the processes of polymerization -depolymerization of microfilaments, bind them to each other and impart contractile properties.
Among these proteins, myosins are of particular importance.
Interaction one of their family, myosin II with actin, underlies muscle contraction, and in non-muscle cells it imparts contractile properties to actin microfilaments - the ability to mechanical stress. This ability plays an extremely important role in all adhesive interactions.

Formation of new actin microfilaments in the cell occurs by their branching from the previous filaments.
For a new microfilament to be able to form, a kind of "seed" is needed. A key role in its formation is played by the Af 2/3 protein complex, which includes two proteins that are very similar to actin monomers.
Being activated, the Af 2/3 complex attaches to the lateral side of the preexisting actin microfilament and changes its configuration, acquiring the ability to attach another actin monomer to itself.
This is how a "seed" appears, initiating the rapid growth of a new microfilament, branching off the lateral side of the old filament at an angle of about 70 °, thereby forming a branched network of new microfilaments in the cell.
The growth of individual filaments soon ends, the filament is disassembled into separate ADP-containing actin monomers, which, after replacing ADP with ATP in them, re-enter the polymerization reaction.
Actin cytoskeleton plays a key role in the attachment of cells to the extracellular matrix and to each other, in the formation of pseudopodia, with the help of which cells can spread and move directionally.
- Return to the section " oncology"
- Methylation of suppressor genes as a cause of hemoblastosis - blood tumors
- Telomerase - synthesis, functions
- Telomere - molecular structure
- What is the telomere effect of posture?
- Alternative ways of telomere lengthening in humans - immortalization
- The importance of telomerase in the diagnosis of tumors
- Cancer Treatments by Influencing Telomeres and Telomerase
- Cell telomerization - does not lead to malignant transformation
- Cell adhesion - the consequences of a violation of adhesive interactions
- Actin microfilaments - structure, function
Microfilaments(thin filaments) - a component of the cytoskeleton of eukaryotic cells. They are thinner than microtubules and are structurally thin protein filaments with a diameter of about 6 nm.
The main protein in their composition is actin... Myosin can also be found in cells. In the bundle, actin and myosin provide movement, although actin alone can do this in the cell (for example, in the microvilli).
Each microfilament consists of two twisted chains, each of which consists of actin molecules and other proteins in smaller quantities.
In some cells, microfilaments form bundles under the cytoplasmic membrane, separate the mobile and immobile parts of the cytoplasm, and participate in endo- and exocytosis.
Also, the functions are to ensure the movement of the entire cell, its components, etc.

Intermediate filaments(they are not found in all eukaryotic cells, they are not found in a number of groups of animals and all plants) differ from microfilaments in a greater thickness, which is about 10 nm.
Microfilaments, their composition and functions
They can be built and destroyed from either end, while thin filaments are polar, they are assembled from the plus end and disassembled from the minus end (just like microtubules).
There are various types of intermediate filaments (differing in protein composition), one of which is found in the cell nucleus.
The protein filaments forming the intermediate filament are antiparallel.
This explains the lack of polarity. At the ends of the filament are globular proteins.

They form a kind of plexus around the nucleus and diverge to the periphery of the cell. Provide the cage with the ability to withstand mechanical stress.
The main protein is actin.
Actin microfilaments.
Microfilaments in general.
Found in all eukaryotic cells.
Location
Microfilaments form bundles in the cytoplasm of mobile animal cells and form a cortical layer (under the plasma membrane).
The main protein is actin.
- Inhomogeneous protein
- Found in different isoforms, encoded by different genes
Mammals have 6 actins: one in skeletal muscles, one in cardiac, two types in smooth, two non-muscle (cytoplasmic) actins = a universal component of any mammalian cells.
All isoforms are similar in amino acid sequences, only the terminal regions are variant (they determine the polymerization rate, DO NOT affect the contraction)
Actin properties:
- M = 42 thousand;
- in monomeric form it has the form of a globule containing an ATP molecule (G-actin);
- actin polymerization => thin fibril (F-actin, is a flat spiral tape);
- actin MFs are polar in their properties;
- at a sufficient concentration, G-actin begins to polymerize spontaneously;
- very dynamic structures that are easy to disassemble and assemble.
During polymerization (+) the end of the microfilament filament quickly binds to G-actin => grows faster
(-) end.
Low concentration of G-actin => F-actin begins to understand.
Critical G-actin concentration => dynamic equilibrium (microfilament has constant length)
Monomers with ATP are attached to the growing end, ATP hydrolysis occurs during polymerization, and the monomers become bound with ADP.
Actin + ATP molecules interact more strongly with each other than ADP-bound monomers.
The stability of the fibrillar system is maintained:
- protein tropomyosin (stiffens);
- filamin and alpha-actinin.
Microfilaments
Form transverse staples between f-actin filaments => a complex three-dimensional network (gives a gel-like state to the cytoplasm);
- Proteins that attach to the ends of the fibrils, preventing disassembly;
- Fimbrin (tie the filaments into bundles);
- Complex with myosins = acto-myosin complex capable of contraction when cleaving ATP.
Functions of microfilaments in non-muscle cells:
Be part of the contractile apparatus;