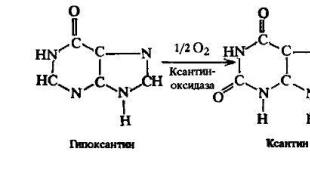
What is the hardest thing on earth. Chemical records. Records for organics
Among the substances always try to select those that have the most extreme degree of a particular property. People have always been attracted to the hardest materials, the lightest or heaviest, light and refractory. We invented the concept of an ideal gas and an ideal black body, and then tried to find natural analogues as close as possible to these models. As a result, a person managed to find or create amazing substances.
1. 
This substance is capable of absorbing up to 99.9% of light, an almost perfect blackbody. It was obtained from specially connected layers of carbon nanotubes. The surface of the resulting material is rough and practically does not reflect light. The areas of application for such a substance are extensive - from superconducting systems to improving the properties of optical systems. For example, through the use of such a material, it would be possible to raise the quality of telescopes and greatly increase the efficiency of solar batteries.
2. 
Few have heard of napalm. But this is only one of the representatives of the class of strong combustible substances. These include styrofoam, and especially chlorine trifluoride. This strongest oxidizing agent can ignite even glass; it reacts violently with almost all inorganic and organic compounds. There are cases when a spilled ton of chlorine trifluoride as a result of a fire burned through the concrete coating of the site and another meter-long gravel-sand cushion deep into 30 centimeters. There were attempts to use the substance as a military poison or rocket fuel, but they were abandoned due to too much danger.
3. 
The strongest poison on earth is also one of the most popular cosmetics. We are talking about botulinum toxins, used in cosmetology under the name botox. This substance is a product of the vital activity of the bacteria Clostridium botulinum and has the greatest molecular weight among proteins. This is the reason for its properties as the most powerful poisonous substance. Enough 0.00002 mg min / l of dry matter to make the affected area deadly for humans for 12 hours. In addition, this substance is perfectly absorbed from the mucous membranes and causes severe neurological symptoms.
4. 
In the depths of the stars, nuclear fires burn, reaching unimaginable temperatures. But man managed to get closer to these figures, having received quark-gluon "soup". This substance has a temperature of 4 trillion degrees Celsius, which is 250,000 times hotter than the sun. It was obtained by colliding gold atoms at almost the speed of light, as a result of which neutrons and protons were melted. True, this substance existed for only a trillionth of one trillionth of a second and occupied one trillionth of a centimeter.
5. 
In this nomination, fluoride-antimony acid becomes the record holder. It is 21,019 times more corrosive than sulfuric acid and can melt through glass and explode when water is added. In addition, it emits deadly toxic fumes.
6. 
Octogen is the most powerful explosive, moreover, resistant to high temperatures. This is what makes it indispensable in military affairs - to create shaped charges, plastites, powerful explosives, fillers for fuses nuclear charges. HMX is also used for peaceful purposes, for example, when drilling high-temperature gas and oil wells, and also as a component of solid rocket fuel. HMX also has an analogue of heptanitrocuban, which has even greater explosive power, but is also more expensive, and therefore is used more in laboratory conditions.

This substance does not have stable isotopes in nature, while generating great amount radioactive radiation. Some of the isotopes polonium-210”, is used to create very light, compact and at the same time very powerful neutron sources. In addition, polonium is used in alloys with certain metals to create heat sources for nuclear installations, in particular, such devices are used in space. At the same time, due to the short half-life of this isotope, it is a highly toxic substance that can cause severe radiation sickness.
8. 
In 2005, German scientists designed a substance in the form of a diamond nanorod. It is a set of diamonds at the nanoscale. Such a substance has the lowest degree of compression and the highest specific gravity known to mankind. In addition, a coating of such a material will have great wear resistance.
9. 
Another creation of specialists from laboratories. It was obtained on the basis of iron and nitrogen in 2010. For now, the details are kept secret, since the previous substance in 1996 could not be reproduced again. But it is already known that the record holder has 18% stronger magnetic properties than the closest analogue. If this substance becomes available on an industrial scale, then we can expect the appearance of the most powerful electromagnetic engines.
10. The strongest superfluidity
We present a selection of chemical records from the Guinness Book of Records.
Due to the fact that new substances are constantly being discovered, this selection is not permanent.
Chemical records for inorganic substances
- The most common element in the earth's crust is oxygen O. Its weight content is 49% of the mass of the earth's crust.
- The rarest element in the earth's crust is astatine At. Its content in the entire earth's crust is only 0.16 g. The second place in terms of rarity is occupied by Fr.
- The most common element in the universe is hydrogen H. Approximately 90% of all atoms in the universe are hydrogen. Helium He is the second most abundant in the universe.
- The strongest stable oxidizing agent is a complex of krypton difluoride and antimony pentafluoride. Due to its strong oxidizing effect (it oxidizes almost all elements to the highest oxidation states, including oxidizing atmospheric oxygen), it is very difficult for it to measure the electrode potential. The only solvent that reacts with it rather slowly is anhydrous hydrogen fluoride.
- Most dense matter on the planet Earth - osmium. The density of osmium is 22.587 g/cm 3 .
- Lithium is the lightest metal. The density of lithium is 0.543 g/cm 3 .
- The densest compound is ditungsten carbide W 2 C. The density of ditungsten carbide is 17.3 g/cm 3 .
- Graphene aerogels are currently the least dense solids. They are a system of graphene and nanotubes filled with air gaps. The lightest of these aerogels has a density of 0.00016 g/cm3. The previous solid with the lowest density is silicon airgel (0.005 g/cm3). Silicon airgel is used in the collection of micrometeorites present in comet tails.
- The lightest gas and, at the same time, the lightest non-metal is hydrogen. The mass of 1 liter of hydrogen is only 0.08988 grams. In addition, hydrogen is also the most fusible non-metal at normal pressure (melting point is -259.19 0 C).
- The lightest liquid is liquid hydrogen. The mass of 1 liter of liquid hydrogen is only 70 grams.
- The heaviest inorganic gas at room temperature is tungsten hexafluoride WF 6 (boiling point is +17 0 C). The density of tungsten hexafluoride as a gas is 12.9 g/l. Among gases with a boiling point below 0 °C, the record belongs to tellurium hexafluoride TeF 6 with a gas density at 25 0 С of 9.9 g/l.
- The most expensive metal in the world is californium Cf. The price of 1 gram of the 252 Cf isotope reaches 500 thousand US dollars.
- Helium He is the substance with the lowest boiling point. Its boiling point is -269 0 C. Helium is the only substance that does not have a melting point at normal pressure. Even at absolute zero, it remains liquid and can only be obtained in solid form under pressure (3 MPa).
- The most refractory metal and the substance with the highest boiling point is tungsten W. The melting point of tungsten is +3420 0 C, and the boiling point is +5680 0 C.
- The most refractory material is an alloy of hafnium and tantalum carbides (1:1) (melting point +4215 0 С)
- The most fusible metal is mercury. The melting point of mercury is -38.87 0 C. Mercury is also the heaviest liquid, its density at 25°C is 13.536 g/cm 3 .
- Iridium is the most resistant metal to acids. Until now, no acid or mixture of them is known in which iridium would dissolve. However, it can be dissolved in alkalis with oxidizing agents.
- The strongest stable acid is a solution of antimony pentafluoride in hydrogen fluoride.
- The hardest metal is chromium Cr.
- The softest metal at 25 0 C is cesium.
- The hardest material is still diamond, although there are already about a dozen substances approaching it in hardness (boron carbide and nitride, titanium nitride, etc.).
- Silver is the most conductive metal at room temperature.
- The lowest speed of sound in liquid helium at 2.18 K is only 3.4 m/s.
- The highest speed of sound in diamond is 18600 m/s.
- The isotope with the shortest half-life is Li-5, which decays in 4.4 10-22 seconds (proton ejection). Because of such a short lifetime, not all scientists recognize the fact of its existence.
- The isotope with the longest measured half-life is Te-128, with a half-life of 2.2 x 1024 years (double β-decay).
- Xenon and cesium have the most number of stable isotopes (36 each).
- The shortest names chemical element have boron and iodine (3 letters each).
- The longest names of a chemical element (eleven letters each) are protactinium Pa, rutherfordium Rf, darmstadtium Ds.
Chemical records for organics
- The heaviest organic gas at room temperature, and the heaviest gas of all at room temperature, is N-(octafluorobut-1-ylidene)-O-trifluoromethylhydroxylamine (b.p. +16 C). Its density as a gas is 12.9 g/l. Among gases with a boiling point below 0°C, the record belongs to perfluorobutane with a gas density at 0°C of 10.6 g/l.
- The most bitter substance is denatonium saccharinate. The combination of denatonium benzoate with the sodium salt of saccharin gave a substance 5 times more bitter than the previous record holder (denatonium benzoate).
- The most non-toxic organic substance is methane. With an increase in its concentration, intoxication occurs due to a lack of oxygen, and not as a result of poisoning.
- The strongest adsorbent for water was obtained in 1974 from a starch derivative, acrylamide and acrylic acid. This substance is able to hold water, the mass of which is 1300 times greater than its own.
- The strongest adsorbent for petroleum products is carbon airgel. 3.5 kg of this substance can absorb 1 ton of oil.
- The most fetid compounds are ethyl selenol and butyl mercaptan - their smell resembles a combination of the smells of rotting cabbage, garlic, onions and sewage at the same time.
- The sweetest substance is N-((2,3-methylenedioxyphenylmethylamino)-(4-cyanophenylimino)methyl)aminoacetic acid (lugduname). This substance is 205,000 times sweeter than a 2% sucrose solution. There are several of its analogues with a similar sweetness. Of industrial substances, the sweetest is talin (a complex of thaumatin and aluminum salts), which is 3,500 to 6,000 times sweeter than sucrose. Recently, neotame has appeared in the food industry with a sweetness 7000 times higher than sucrose.
- The slowest enzyme is nitrogenase, which catalyzes the assimilation of atmospheric nitrogen by nodule bacteria. The full cycle of transformation of one nitrogen molecule into 2 ammonium ions takes one and a half seconds.
- The organic substance with the highest nitrogen content is either bis(diazotetrazolyl)hydrazine C2H2N12, containing 86.6% nitrogen, or tetraazidomethane C(N3)4, containing 93.3% nitrogen (depending on whether the latter is considered organic or not) . These explosives are extremely sensitive to impact, friction and heat. Of inorganic substances, the record certainly belongs to gaseous nitrogen, and of compounds, to hydrazoic acid HN 3 .
- The longest chemical name has 1578 English characters and is a modified nucleotide sequence. This substance is called: Adenosene. N--2'-O-(tetrahydromethoxypyranyl)adenylyl-(3'→5')-4-deamino-4-(2,4-dimethylphenoxy)-2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3'→5 ')-4-deamino-4-(2,4-dimethylphenoxy)-2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3'→5')-N--2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3 '→5')-N--2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3'→5')-N--2'-O-(tetrahydromethoxypyranyl)guanylyl-(3'→5')-N- -2′-O-(tetrahydromethoxypyranyl)guanylyl-(3'→5′)-N--2′-O-(tetrahydromethoxypyranyl)adenylyl-(3′→5′)-N--2′-O-(tetrahydromethoxypyranyl )cytidylyl-(3'→5′)-4-deamino-4-(2,4-dimethylphenoxy)-2′-O-(tetrahydromethoxypyranyl)cytidylyl-(3′→5′)-4-deamino-4-( 2,4-dimethylphenoxy)-2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3'→5')-N--2'-O-(tetrahydromethoxypyranyl)guanylyl-(3'→5')-4-deamino- 4-(2,4-dimethylphenoxy)-2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3'→5')-N--2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3'→5')-N --2'-O-(tetrahydromethoxypyranyl)cytidylyl-(3'→5')-N--2'-O-(tetrahydromethoxypyranyl)adenylyl-(3'→5')-N--2'-O-( tetrahydro methoxypyranyl)cytidylyl-(3'→5′)-N--2′-O-(tetrahydromethoxypyranyl)cytidylyl-(3′→5′)-N--2′,3′-O-(methoxymetylene)-octadecakis( 2-chlorophenyl)ester. 5'-.
- The longest chemical name possesses DNA isolated from human mitochondria and consisting of 16569 base pairs. The full name of this compound contains about 207,000 characters.
- The system of the largest number of immiscible liquids, again stratified into components after mixing, contains 5 liquids: mineral oil, silicone oil, water, benzyl alcohol and N-perfluoroethylperfluoropyridine.
- The densest organic liquid at room temperature is diiodomethane. Its density is 3.3 g/cm3.
- The most refractory individual organic matter are some aromatic compounds. Of the condensed ones, this is tetrabenzheptacene (melting point +570 C), of the non-condensed ones, p-septiphenyl (melting point of +545 C). Exists organic compounds for which the melting point is not precisely measured, for example, for hexabenzocoronene, its melting point is indicated to be above 700 C. The product of thermal crosslinking of polyacrylonitrile decomposes at a temperature of about 1000 C.
- The organic substance with the highest boiling point is hexatriaconylcyclohexane. It boils at +551°C.
- The longest alkane is nonacontatrictane C390H782. It was specially synthesized to study the crystallization of polyethylene.
- The longest protein is the muscle protein titin. Its length depends on the type of living organism and localization. Mouse titin, for example, has 35213 amino acid residues (molecular weight 3906488 Da), human titin has a length of up to 33423 amino acid residues (molecular weight 3713712 Da).
- The longest genome is the genome of the plant Paris japonica (Paris japonica). It contains 150,000,000,000 base pairs - 50 times more than in humans (3,200,000,000 base pairs).
- The largest molecule is the DNA of the first human chromosome. It contains about 10,000,000,000 atoms.
- The individual explosive with the highest rate of detonation is 4,4'-dinitroazofuroxan. Its measured detonation velocity was 9700 m/s. According to unverified data, ethyl perchlorate has an even higher detonation speed.
- The individual explosive with the highest heat of explosion is ethylene glycol dinitrate. Its heat of explosion is 6606 kJ/kg.
- The strongest organic acid is pentacyanocyclopentadiene.
- Perhaps the strongest base is 2-methylcyclopropenyllithium. The strongest nonionic base is phosphazene, which has a rather complex structure.
Among the curiosities hidden in the depths of the universe, a small star near Sirius will probably forever preserve one of the significant places. This star is made of matter 60,000 times heavier than water! When we pick up a glass of mercury, we are surprised by its heaviness: it weighs about 3 kg. But what would we say about a glass of matter weighing 12 tons and requiring a railway platform to transport? This seems absurd, and yet such is one of the discoveries of modern astronomy.
This discovery has a long and highly instructive history. It has long been observed that the resplendent Sirius makes his own motion among the stars, not in a straight line, like most other stars, but in a strange tortuous path. To explain these features of its movement, the famous astronomer Bessel suggested that Sirius was accompanied by a satellite, which “perturbed” its movement with its attraction. This was in 1844 - two years before Neptune was discovered "at the tip of a pen." And in 1862, after Bessel's death, his guess was fully confirmed, since the suspected satellite of Sirius was seen through a telescope.
The satellite of Sirius - the so-called "Sirius B" - orbits about main star at 49 years at a distance 20 times greater than the Earth around the Sun (i.e., about the distance of Uranus). This is a weak star of the eighth or ninth magnitude, but its mass is very impressive, almost 0.8 of the mass of our Sun. At the distance of Sirius, our Sun would have to shine as a star of magnitude 1.8; therefore, if the satellite of Sirius had a surface reduced in comparison with the solar one in accordance with the ratio of the masses of these luminaries, then at the same temperature it would have to shine like a star of about the second magnitude, and not the eighth or ninth. Astronomers originally explained such a weak brightness by the low temperature on the surface of this star; it was considered as a cooling sun, covered with an already solid crust.
But this assumption turned out to be wrong. It was possible to establish that the modest satellite of Sirius is not a fading star at all, but, on the contrary, belongs to stars with a high surface temperature, much higher than that of our Sun. This completely changes things. The weak brightness must therefore be attributed only to the small size of the surface of this star. It is calculated that it sends out 360 times less light than the Sun; this means that its surface must be at least 360 times smaller than the sun, and the radius must be j/360, i.e., 19 times less than the sun. From this we conclude that the volume of the satellite of Sirius should be less than 6800th of the volume of the Sun, while its mass is almost 0.8 of the mass of the daylight. This alone speaks of the high density of the matter of this star. A more accurate calculation gives for the diameter of the planet only 40,000 km, and therefore, for the density - the monstrous number that we gave at the beginning of the section: 60,000 times the density of water.
“Prick up your ears, physicists: an invasion is being planned into your area,” Kepler’s words come to mind, spoken by him, however, on a different occasion. Indeed, no physicist could imagine anything like this until now. Under normal conditions, such a significant compaction is completely unthinkable, since the gaps between normal atoms in solids are too small to allow any noticeable compression of their substance. The situation is different in the case of "mutilated" atoms that have lost those electrons that circled around the nuclei. The loss of electrons reduces the diameter of an atom by several thousand times, almost without reducing its weight; the naked nucleus is about as many times smaller than a normal atom as a fly is smaller than a large building. Shifted by the monstrous pressure prevailing in the bowels of the stellar ball, these reduced atoms-nuclei can approach a thousand times closer than normal atoms, and create a substance of that unheard-of density, which is found on the satellite of Sirius.
After what has been said, it will not seem incredible the discovery of a star whose average density of matter is another 500 times greater than that of the matter of the previously mentioned star Sirius B. We are talking about a small star of the 13th magnitude in the constellation Cassiopeia, discovered at the end of 1935. no larger than Mars and eight times smaller than the globe, this star has a mass almost three times the mass of our Sun (more precisely, 2.8 times). In ordinary units, the average density of its substance is expressed as 36,000,000 g/cm3. This means that 1 cm3 of such a substance would weigh 36 tons on Earth. This substance, therefore, is almost 2 million times denser than gold.
A few years ago, of course, scientists would have considered the existence of a substance millions of times denser than platinum unthinkable. The abysses of the universe hide, probably, many more such wonders of nature.
Since time immemorial, people have been actively using various metals. After studying their properties, the substances took their rightful place in the table of the famous D. Mendeleev. Until now, the disputes of scientists regarding the question of which metal should be given the title of the heaviest and densest in the world have not subsided. On the scales are two elements of the periodic table - iridium, as well as osmium. What are they interesting, read on.
For centuries, people have been studying the beneficial properties of the most common metals on the planet. Science stores the most information about gold, silver and copper. Over time, mankind got acquainted with iron, lighter metals - tin and lead. In the world of the Middle Ages, people actively used arsenic, and diseases were treated with mercury.
Thanks to rapid progress, today the heaviest and densest metals are considered not one element of the table, but two at once. Osmium (Os) is located at number 76, and iridium (Ir) at number 77, substances have the following density indicators:
- osmium is heavy due to its density of 22.62 g/cm³;
- iridium is not much lighter - 22.53 g / cm³.
Density refers to physical properties metals, it is the ratio of the mass of a substance to its volume. Theoretical calculations of the density of both elements have some errors, so both metals are now considered to be the heaviest.
For clarity, you can compare the weight of an ordinary cork with the weight of a cork made of the heaviest metal in the world. To balance scales with an osmium or iridium stopper, more than a hundred ordinary stoppers will be required.
History of discovery of metals
Both elements were discovered at the dawn of the 19th century by Smithson Tennant. Many researchers of that time were studying the properties of raw platinum, processing it with "royal vodka". Only Tennant was able to detect two chemicals in the resulting sediment:
- the sedimentary element with a persistent smell of chlorine, the scientist called osmium;
- a substance with a changing color is called iridium (rainbow).
Both elements were represented by a single alloy, which the scientist managed to separate. Further study of platinum nuggets was undertaken by the Russian chemist K. Klaus, who carefully studied the properties of sedimentary elements. The difficulty of determining the heaviest metal in the world lies in the low difference in their density, which is not a constant value.

Vibrant characteristics of the densest metals
Experimentally obtained substances are a powder, rather difficult to process, forging metals requires very high temperatures. The most common form of the commonwealth of iridium with osmium is an alloy of osmic iridium, which is mined in platinum deposits, gold beds.
Iron-rich meteorites are considered the most common place to find iridium. Native osmium is not found in the natural world, only in commonwealth with iridium and other components of the platinum group. The deposits often contain sulfur compounds with arsenic.
Features of the heaviest and most expensive metal in the world
Among the elements of Mendeleev's periodic table, osmium is considered the most expensive. The silvery metal with a bluish tint belongs to the platinum group of noble chemical compounds. The most dense, but very fragile metal does not lose its luster under the influence of high temperature indicators.

Specifications
- Element #76 Osmium has an atomic mass of 190.23 amu;
- A substance molten at 3033°C will boil at 5012°C.
- The heaviest material has a density of 22.62 g/cm³;
- The structure of the crystal lattice has a hexagonal shape.
Despite the amazingly cold sheen of a silvery sheen, osmium is not suitable for jewelry production due to its extreme toxicity. To melt the jewelry, it would take a temperature like on the surface of the Sun, because the densest metal in the world is destroyed by mechanical action.
Turning into powder, osmium interacts with oxygen, reacts with sulfur, phosphorus, selenium, the reaction of the substance with aqua regia is very slow. Osmium does not possess magnetism, alloys tend to oxidize and form cluster compounds.

Where apply
The heaviest and incredibly dense metal has high wear resistance, so adding it to alloys significantly increases their strength. The use of osmium is mainly associated with the chemical industry. In addition, it is used for the following needs:
- manufacture of containers intended for storage of nuclear fusion waste;
- for the needs of rocket science, weapons production (warheads);
- in the watch industry for the manufacture of mechanisms of branded models;
- for the manufacture of surgical implants, parts of pacemakers.
Interestingly, the densest metal is considered the only element in the world that is not subject to the aggression of the “hellish” mixture of acids (nitric and hydrochloric). Aluminum combined with osmium becomes so ductile that it can be drawn without breaking.
Secrets of the rarest and densest metal in the world
The fact that iridium belongs to the platinum group endows it with the property of immunity to treatment with acids and their mixtures. In the world, iridium is obtained from anode slimes in copper-nickel production. After processing the sludge with aqua regia, the precipitate is calcined, resulting in the extraction of iridium.

Specifications
The hardest silver-white metal has the following group of properties:
- element of the periodic table Iridium No. 77 has an atomic mass of 192.22 amu;
- a substance molten at 2466°C will boil at 4428°C;
- the density of molten iridium is within 19.39 g/cm³;
- element density at room temperature - 22.7 g / cm³;
- the crystal lattice of iridium is associated with a face-centered cube.
Heavy iridium does not change under the influence of ordinary air temperature. The result of calcination under the influence of heating at certain temperatures is the formation of polyvalent compounds. The powder of fresh sediment of iridium black lends itself to partial dissolution with aqua regia, as well as with a solution of chlorine.

Application area
Although Iridium is a precious metal, it is rarely used in jewelry. An element that is difficult to process is in great demand in the construction of roads, the production of automotive parts. Alloys with the densest metal that is not susceptible to oxidation are used for the following purposes:
- production of crucibles for laboratory experiments;
- production of special mouthpieces for glassblowers;
- covering the tips of nibs and refills of ballpoint pens;
- production of durable spark plugs for cars;
Alloys with iridium isotopes are used in welding production, in instrumentation, and for growing crystals as part of laser technology. The use of the heaviest metal has made it possible to carry out laser vision correction, crushing of kidney stones and other medical procedures.
Although Iridium is devoid of toxicity and is not harmful to biological organisms, in the natural environment you can meet its dangerous isotope - hexafluoride. Inhalation of poisonous vapors leads to instant suffocation and death.
Places of natural occurrence
The deposits of the densest metal in the natural world, Iridium, are minuscule, much smaller than those of platinum. Presumably, the heaviest substance has shifted to the core of the planet, so the volume of industrial production of the element is small (about three tons per year). Iridium alloy products can last up to 200 years, jewelry will become more durable.

Nuggets of the heaviest metal with an unpleasant odor, Osmium, cannot be found in nature. In the composition of minerals, traces of osmic iridium can be found along with platinum and palladium, ruthenium. Deposits of osmic iridium have been explored in Siberia (Russia), some states of America (Alaska and California), Australia and South Africa.
If deposits of platinum are found, it will be possible to isolate osmium with iridium to strengthen and strengthen the physical or chemical compounds of various products.
This basic list of ten elements is the "heaviest" in terms of density per cubic centimeter. However, note that density is not mass, it simply indicates how tightly packed the mass of a body is.
Now that we understand this, let's take a look at the heaviest in the entire universe known to mankind.
10. Tantalum
Density per 1 cm³ - 16.67 g
Tantalum has an atomic number of 73. This blue-gray metal is very hard and also has a super high melting point.
9. Uranium (Uranium) 
Density per 1 cm³ - 19.05 g
Discovered in 1789 by the German chemist Martin H. Klaprot, the metal did not become real uranium until almost a hundred years later, in 1841, thanks to the French chemist Eugène Melchior Peligot.
8. Wolframium 
Density per 1 cm³ - 19.26 g
Tungsten exists in four different minerals and is also the heaviest of all the elements that play an important biological role.
7. Gold (Aurum) 
Density per 1 cm³ - 19.29 g
They say money doesn't grow on trees, which can't be said about gold! Small traces of gold have been found on the leaves of eucalyptus trees.
6. Plutonium (Plutonium) 
Density per 1 cm³ - 20.26 g
Plutonium exhibits a colorful oxidation state in aqueous solution, and can also spontaneously change oxidation states and colors! This is a real chameleon among the elements.
5. Neptunium 
Density per 1 cm³ - 20.47 g
Named after the planet Neptune, it was discovered by Professor Edwin McMillan in 1940. It also became the first discovered synthetic transuranium element from the actinide family.
4. Rhenium 
Density per 1 cm³ - 21.01 g
The name of this chemical element comes from the Latin word "Rhenus", which means "Rhine". It was discovered by Walter Noddack in Germany in 1925.
3. Platinum (Platinum) 
Density per 1 cm³ - 21.45 g
One of the most precious metals on this list (along with gold), and is used to make just about everything. As a strange fact: all the mined platinum (down to the last particle) could fit in a medium-sized living room! Not much, really. (Try putting all the gold in it.)
2. Iridium (Iridium)
Density per 1 cm³ - 22.56 g
Iridium was discovered in London in 1803 by the English chemist Smithson Tennant (Smithson Tennant) along with osmium: the elements were present in natural platinum as impurities. Yes, iridium was discovered purely by accident.
1. Osmium
Density per 1 cm³ - 22.59 g
There is nothing heavier (per cubic centimeter) than osmium. The name of this element comes from ancient Greek word"osme", which means "smell", since the chemical reactions of its dissolution in acid or water are accompanied by an unpleasant, persistent odor.