Carboxylic acids. Carboxylic acids - presentation presentation for a chemistry lesson (grade 10) on the topic Carboxylic acids in nature presentation
Presentation on the topic: Application of carboxylic acids Work performed by: Borisenko Gleb G. Zhigulevsk
Carboxylic acids Carboxylic acids are organic compounds whose molecules contain one or more functional carboxyl groups -COOH.
Classification Depending on the radical associated with the carboxyl, the following groups of carboxylic acids are distinguished: aromatic (benzoic acid) aliphatic (including saturated (caproic acid) and unsaturated (acrylic acid)) alicyclic (quinic acid) heterocyclic (nicotinic acid). According to the number of carboxyl groups, acids can be: monobasic (acetic acid), dibasic (oxalic acid), polybasic (citric acid). When other functional groups are introduced into acid molecules (for example, -OH, =CO, -NH 2, etc.), hydroxy-, keto-, amino acids and other classes of compounds are formed.
Monobasic saturated carboxylic acids Formic acid is found in ant secretions, nettles, bee venom, and pine needles. Acetic acid is a product of acetic acid fermentation. Valeric acid is found in valerian root. Butyric acid is formed when butter goes rancid. Pelargonic acid is found in the volatile oil of rosea pelargonium and other plants of the geranium family. Palmitic acid is most easily isolated from palm oil, which is extracted from coconut kernels (copra). Stearic acid is one of the most important fatty acids and makes up the main part of most vegetable and animal fats.
Formic acid Formic acid (methanoic acid) is the first representative in a series of saturated monobasic carboxylic acids. Registered as a food additive under the designation E236. Application In the food industry, formic acid (E236) is mainly used as an additive in the production of canned vegetables. It slows down the development of pathogenic environments and molds in canned and pickled vegetables. It is also used in the production of soft drinks, as part of fish marinades and other acidic fish products. In addition, it is often used to disinfect wine and beer barrels. In medicine, formic acid is used as an antiseptic, cleansing and analgesic, and in some cases as a bactericidal and anti-inflammatory.
Acetic acid Acetic acid (ethanoic acid) is an organic substance with the formula CH 3 COOH. Weak, limiting monobasic carboxylic acid. Salts and esters of acetic acid are called acetates. CH 3 COOH Application Acetic acid, the concentration of which is close to 100%, is called glacial. A 70-80% aqueous solution of acetic acid is called vinegar essence, and 3-15% is called vinegar. Aqueous solutions of acetic acid are widely used in the food industry (food additive E260) and household cooking, as well as in canning. Acetic acid is used to obtain medicinal and fragrant substances, as a solvent (for example, in the production of cellulose acetate, acetone). It is used in printing and dyeing.
Valeric acid Valeric acid (pentanoic acid) C 4 H 9 COOH is a monobasic saturated carboxylic acid, a colorless liquid with an unpleasant odor. Salts and esters of valeric acid are called valerates. Application Valeric acid is used for the synthesis of medicinal substances: validol, bromural, etc., as well as for the chemical synthesis of racemates of the amino acid valine. In the food industry, isoamyl ester of isovaleric acid (apple essence) is used, and ethyl and pentyl ester of valeric acid are also used.
Butyric acid Butyric acid (butanoic acid) C 3 H 7 COOH is a colorless liquid with a pungent odor of rancid oil, which has one carboxyl group in its structure and belongs to monobasic (short-chain) saturated fatty acids (SCFA). Salts and esters of butyric acid are called butyrates. Application Butyric acid and isobutyric acid are used as an extractant of alkaline earth elements (Ca, Sr, Mg, Ba) when purifying rare earth elements from them, for deliming (removing Ca salts), for example, when decalcifying leather; in the synthesis of fragrant substances for perfumery (for example, geranyl butyrate, citronellyl butyrate), flavoring agents in the food industry (methyl butyrate has the smell of apples, isoamyl butyrate has the smell of pears), plasticizers for varnishes based on cellulose ethers (for example, glyceryl tributyrate), emulsifiers (for example, 2-hydroxyethylbutyrate), cellulose acetobutyrate, which serves as the basis for weather-resistant coatings, butyrylcholine halides (substrates for the detection of cholinesterase).
Pelargonic acid Pelargonic acid (Nonanoic acid) C 8 H 17 COOH is a monobasic saturated carboxylic acid. It is an oily liquid that solidifies upon cooling into a leafy-crystalline mass that melts at 12.5 °C; t boil 253-254 °C. Application Pelargonic acid is used in the production of polyester alkyd resins, dyes, stabilizers; Substituted pelargonic acids are used as biostimulants, esters are used as fragrant substances, for example, ethyl pelargonate has the smell of roses.
Palmitic acid Palmitic acid (Hexadecanoic acid) CH 3 (CH 2) 14 COOH is the most common monobasic saturated carboxylic acid (fatty acid) in nature. Salts and esters of palmitic acid are called palmitates. Application Palmitic acid is used in the production of stearin, napalm, detergents and cosmetics, lubricating oils and plasticizers. Calcium palmitate is used as a component of compositions for hydrophobization of fabrics, leather, wood, and as an emulsifier in cosmetic preparations. Sodium palmitate - as an emulsifier, component of laundry and toilet soaps, cosmetics;
Stearic acid Stearic acid (octadecanoic acid) is a monobasic carboxylic acid of the aliphatic series, corresponding to the formula C 18 H 36 O 2, or CH 3 (CH 2) 16 COOH. White crystals, insoluble in water and soluble in diethyl ether. Stearic acid was discovered in lard in 1816 by the French chemist Chevrel. Application Widely used in the cosmetics industry: sodium stearate is one of the main components of soap, stearic acid itself is included in many cosmetics. It is used in the production of candles and as a softener in the production of rubber. Sodium, calcium, and lead stearates are used as components of greases.
“Examples of carboxylic acids” - Chemical properties of carboxylic acids. Formic acid. Indicator. They form ethers. Study the structure. Oxalic acid. Acetic acid. Lemon acid. These are organic substances. Stearic acid. Acids. Classification of carboxylic acids. Valeric acid. Carboxylic acids.
“Carboxylic acids and their properties” - Organic substances. Discovery of acids. Carboxylic acids. Name the substance. Interesting historical facts related to organic acids. Dicarboxylic acids. Which acid is stronger? Physical properties of carboxylic acids. R-COOH. Formic acid was first isolated in the 17th century. Acid names.
“Chemical properties of carboxylic acids” - Chemical properties of inorganic acids. Salicylic acid. Formic acid. Carboxylic acids. Oxalic acid. The structure of the carboxyl group. General properties of carboxylic acids. Functional group. Task. Chemical properties of carboxylic acids. Chemical properties. Name of carboxylic acids.
“Limit monobasic carboxylic acids” - Carboxylic acids. Physical properties. Structure and nomenclature of saturated carboxylic acids. Saturated monobasic carboxylic acids. Types of isomerism. History of discovery. Gaseous substances. Name the carboxylic acids. Formic acid. Carbon atom. Monobasic carboxylic acids. Trivial names.
“Classes of carboxylic acids” - Oxygen-containing organic compounds. Benzoic acid. Statements. Classification of carboxylic acids by the number of functional groups. Classification of carboxylic acids according to the nature of the hydrocarbon radical. Salicylic acid. Lemon acid. Physical properties of carboxylic acids. Production of organic compounds.
“Saturated carboxylic acids” - Use the formulas to carry out a chain of transformations. Name the carboxylic acids. Test your knowledge of carboxylic acids. Nomenclature of esters. Structure of monobasic carboxylic acids. Preparation of carboxylic acids. Carbon atom. Definition. React with basic oxides. Ethane.
There are 19 presentations in total
1. Find out the functional group and general formula of carboxylic acids.
2. Formulate a definition.
3. Study the classification of carboxylic acids.
4. Master nomenclature skills.
5. Consider the physical and chemical properties of the most important carboxylic acids.
6. Find out the areas of application of some carboxylic acids.
Download:
Preview:
To use presentation previews, create a Google account and log in to it: https://accounts.google.com
Slide captions:
Tasks 1. Find out the functional group and general formula of carboxylic acids. 2. Formulate a definition. 3. Study the classification of carboxylic acids. 4. Master nomenclature skills. 5. Consider the physical and chemical properties of the most important carboxylic acids. 6. Find out the areas of application of some carboxylic acids.
All carboxylic acids have a functional group - C OH O Carbonyl group Hydroxyl group Carboxyl group General formula R C OH O C n H 2n +1 C Or for saturated monobasic acids O OH? What are carboxylic acids called? Carboxylic acids are organic compounds whose molecules contain a carboxyl group, COOH, bonded to a hydrocarbon radical. ? How are carboxylic acids genetically related to the previously studied class of aldehydes? R C O H + [O] R C O OH [O]= KM nO 4, K 2 Cr 2 O 7+ H 2 SO 4 conc.
Classification of carboxylic acids Depending on the number of carboxyl groups Monobasic Dibasic (acetic) (oxalic) CH 3 C C - CO OH Polybasic (citric) O OH O HO N 2 C - C O OH HC - C O OH H 2 C - C O OH Depending on the nature of the radical Saturated (propionic) O CH 3- CH 2- C OH Unsaturated (acrylic) O CH 2 =CH-C OH Aromatic (benzoic) CO OH Based on the content of C atoms: C 1 -C 9 - lower, With 10 or more - higher
Classify the proposed acids 1) C H 3 - CH 2 - CH 2 - CH 2 - COOH 2) HOOC - CH 2 - CH 2 - COOH 3) COOH 4) CH 3 - (C H 2) 7 - CH = CH - ( CH 2) 7 - COOH 5) HOOC - CH 2 - CH - CH 2 - COOH COOH CH 3 1. Monobasic, limiting, lower 2. Dibasic, limiting, lower 3. Monobasic, limiting, lower 4. Monobasic, unsaturated, highest 5.Polybasic, ultimate, lowest
NOMENCLATURE OF CARBOXYLIC ACIDS ALKANE + OH + OHIC ACID METHANE OHIC ACID (FOMIC ACID)
CH3 – COOH 1 2 ETHANIC ACID (ACETIC ACID) CH3 – CH2 – CH2 - COOH 1 2 3 4 BUTANIC ACID (BUTRYAL ACID)
CH3 – CH2 – CH2 – CH2 – COOH PENTANIC ACID (VALERIAN ACID) 1 2 3 4 5 HOOC – COOH ETHANIC ACID (OXALIC ACID) 1 2
Homologous series of carboxylic acids Chemical formula Systematic name of the acid Trivial name of the acid Name of the acidic residue HCOO H Formic at CH3COO N Acetic acetate CH3CH2COO N Propionic Propion at CH3CH2CH2COO N Butyric at CH3CH2CH2CH2COO N Valeric valerin at CH3-( CH2)4–COO N Capron capron at CH3-(CH2)8 – COO N capric capric at CH3-(CH2)14 – COO N Palmitic palmitate at CH3-(CH2)16- COO N Stearic stearate Methane Ethane Propane Butane Pentane Hexane Decane Hexadecane Octadecane
Algorithm for naming carboxylic acids: 1. We find the main chain of carbon atoms and number it, starting with the carboxyl group. 2. We indicate the position of the substituents and their name(s). 3. After the root, indicating the number of carbon atoms in the chain, comes the suffix “-ic” acid. 4. If there are several carboxyl groups, then a numeral is placed before “- ova” (-di, - three...) Example: 3-methyl butane + -ova = 3-methyl butanoic acid acid
GIVE THE NAME OF THE SUBSTANCES ACCORDING TO THE INTERNATIONAL NOMENCLATURE (2 – METHYL PROPANE ACID) CH3 – CH – COOH 2. CH3 – CH2 – CH – CH – COOH 3. CH3 – CH = CH – CH – COOH 4. HOOS – CH2 – CH – COOH (2, 3 – DIMETHYL PENTANIC ACID) (2 – METHYL PENTANIC ACID) (2 – ETHYL BUTANIC ACID) CH3 CH3 CH3 CH3 C 2 H 5
: 1 . Select the root of the word based on which write down the carbon skeleton in the composition, which includes a carboxyl group. 2. We number the carbon atoms, starting with the carboxyl group. 3. We indicate substituents according to numbering. 4. It is necessary to add the missing hydrogen atoms (carbon is tetravalent). 5. Check that the formula is written correctly. 2-methyl butanoic acid. Example: Algorithm for writing formulas of carboxylic acids
PHYSICAL PROPERTIES C 1 – C 3 Liquids with a characteristic pungent odor, highly soluble in water C 4 – C 9 Viscous oily liquids with an unpleasant odor, poorly soluble in water C 10 and more Odorless solids, insoluble in water
Physical state liquid Color colorless transparent liquid Odor pungent acetic Solubility in water is good Boiling point 118 º C Melting point 17 º C Physical properties of acetic acid:
Lower carboxylic acids are liquids; higher - solid substances. The greater the relative molecular weight of the acid, the less its odor. With an increase in the relative molecular weight of the acid, solubility decreases. Dependence of the physical properties of carboxylic acids on the structure of the molecule: The homologous series of aldehydes begins with two gaseous substances (at room temperature), and there are no gases among carboxylic acids. What is this connected with?
Chemical properties of carboxylic acids I. Common with inorganic acids Soluble carboxylic acids dissociate in aqueous solutions: CH 3 – COOH CH 3 – COO + H + Is the medium acidic? How will the color of indicators change in an acidic environment? Litmus (violet) - turns red Methyl orange - turns pink Phenophthalein - does not change color 2. Interaction with metals in the electrochemical voltage series up to hydrogen: 2CH 3 - COOH + M g Acetic acid (CH 3 -COO) 2 M g Magnesium acetate + H 2 2CH 3 - COOH + Zn Acetic acid (CH 3 –COO) 2 Zn Zinc acetate + H 2 When a metal reacts with a carboxylic acid solution, hydrogen and salt are formed
3. Interaction with basic oxides: 2 CH 3 – COOH + C u O Acetic acid t (CH 3 – COO) 2 C u Copper acetate + H 2 O 4. Interaction with metal hydroxides (neutralization reaction) CH 3 – COOH + HO – Na Acetic acid CH 3 COO Na Sodium acetate + H 2 O 5. Interaction with salts of weaker and volatile acids (for example, carbonic, silicic, hydrogen sulfide, stearic, palmitic...) 2CH 3 – COOH Acetic acid + Na 2 CO 3 sodium carbonate 2CH 3 COO Na Sodium acetate + H 2 CO 3 CO 2 H 2 O 2 CH3 – COOH + Cu(OH) 2 Acetic acid (CH3COO) 2 Cu Copper acetate + H2O
Specific properties of formic acid “Silver Mirror” reaction H- CO OH + Ag 2 O t 2Ag + H 2 CO 3 CO 2 H 2 O Formic acid Silver oxide silver
Occurrence in nature and use of carboxylic acids Formic acid (Methanoic acid) - Chemical formula CH2O2, or HCOOH. - Formic acid was discovered in the acidic secretions of red ants in 1670 by the English naturalist John Ray. Formic acid is also present in the finest nettle hairs, in bee venom, pine needles, and is found in small quantities in various fruits, tissues, organs, and animal and human secretions.
Question: Why can’t you wet the area of an ant bite or nettle sting with water? This only leads to increased pain. Why does the pain subside if the wounded area is moistened with ammonia? What else can be used in this case? When formic acid is dissolved in water, a process of electrolytic dissociation occurs: HCOOH HCOO + H As a result, the acidity of the environment increases, and the process of skin corrosion intensifies. In order for the pain to subside, you need to neutralize the acid, for which you need to use solutions that have an alkaline reaction, for example, a solution of ammonia. HCOOH + N H4OH HCOO NH4 + H2O or HCOOH + NaHCO3 HCOO Na + CO2 + H2O
Acetic acid (ethanoic acid) This is the first acid obtained and used by man. “Born” more than 4 thousand years ago in Ancient Egypt. At the turn of the 17th – 18th centuries in Russia it was called “sour moisture”. The first time we got it was during the souring of wine. The Latin name is Acetum acidum, hence the name of the salts - acetates. Glacial acetic acid at temperatures below 16.8 º C hardens and becomes like ice - Acetic essence - 70% acid solution. - Table vinegar - 6% or 9% acid solution. Acetic acid - found in animal secretions (urine, bile, feces), in plants (in particular, green leaves), in sour milk and cheese; - formed during fermentation, rotting, souring of wine and beer, and during the oxidation of many organic substances;
Application of acetic acid - Aqueous solutions of acetic acid are widely used in the food industry (food additive E-260) and household cooking, as well as in canning; in the production of: drugs, e.g. aspirin; artificial fibers, e.g. silk acetate; indigo dyes, non-flammable film, organic glass; varnish solvents; chemical plant protection products, plant growth stimulants; Sodium acetate CH3COO N a is used as a preservative for blood intended for transfusion; Potassium acetate CH3COOK – as a diuretic; Lead acetate (CH3COO) 2 Pb - for determining sugar in urine; Acetates of iron (III) (CH3COO)3Fe, aluminum (CH3COO) 3Al and chromium (III) (CH3COO) 3Cr are used in the textile industry for mordant dyeing; Copper (II) acetate (CH3COO) 2 C u is part of the preparation for controlling plant pests, the so-called Parisian green;
Use of acetic acid The use of alcohol vinegar in cosmetology is known. Namely, to give softness and shine to hair after perm and permanent coloring. To do this, it is recommended to rinse your hair with warm water with the addition of alcohol vinegar (3-4 tablespoons of vinegar per 1 liter of water). In folk medicine, vinegar is used as a nonspecific antipyretic. for headaches using lotions. for insect bites using compresses. indispensable in the production of perfume products Did you know that - If you need to unscrew a rusty nut, it is recommended to put a rag soaked in acetic acid on it in the evening? It will be much easier to unscrew this nut in the morning. - During the day, 400 g of acetic acid is formed in the body? This would be enough to make 8 liters of ordinary vinegar
Of all the acids, It is, of course, the prima. Present everywhere, both visibly and invisibly. It is present in animals and plants, Technology and medicine are with it forever. Its offspring are acetates - very necessary “guys”. The well-known aspirin, like a good gentleman, reduces the patient’s fever and restores health. This is copper acetate. He is a friend and brother to the plants, Kills their enemies. Acid still has some use - It dresses us in acetate silk. And whoever loves dumplings has known vinegar for a long time. There is also the question of cinema: Well, everyone needs to know that without acetate film we cannot see cinema. Of course there are other uses. And you know them without a doubt. But the main thing that is said, friends, is “Bread of the industry”: acetic acid. CH 3 COOH CH3COOH Aqueous solution of copper acetate
Citric acid COOH HOOC – CH 2 – C – CH 2 – COOH OH Oxalic acid HOOC –– COOH Formic acid H –– COOH Acetylsalicylic acid COOH OCOCH 3 Tartaric acid HOOC – CH - CH –– COOH OH OH Lactic acid CH 3 - CH –– COOH OH Malic acid HOOC – CH - CH 2 –– COOH OH Succinic acid HOOC – CH 2 - CH 2 –– COOH Benzoic acid COOH Ascorbic acid HO OH H =O HOH 2 C-NONS O Acetic acid H 3 C – – COOH Carboxylic acids in order of increasing acidity
Conclusions 1. Carboxylic acids are organic compounds whose molecules contain a carboxyl group - COOH, associated with a hydrocarbon radical. 2.Carboxylic acids are classified: by basicity (mono-, di- and polybasic) by hydrocarbon radical (saturated, unsaturated and aromatic) by the content of C atoms (lower and higher) 3. The name of a carboxylic acid consists of the name of an alkane + ovalic acid. 4. As the molecular weight of a carboxylic acid increases, the solubility and strength of the acid decreases. 5. Like inorganic acids, soluble carboxylic acids dissociate in an aqueous solution, forming hydrogen ions and changing the color of the indicator. They react with metals (up to H), basic and amphoteric oxides and hydroxides, and salts of weaker acids, forming salts. 6 Widely distributed in nature and have great practical importance for humans.
Homework § 14, exercises No. 6 exercise No. 9 Queen Cleopatra, on the advice of the court physician, dissolved the largest pearl known to jewelers in vinegar, and then took the resulting solution for some time. What reaction did Cleopatra make? What connection was she taking? 3. Prepare a report on higher carboxylic acids
Thank you for your attention


Tasks 1. Find out the functional group and general formula of carboxylic acids. 2. Formulate a definition. 3. Study the classification of carboxylic acids. 4. Master nomenclature skills. 5. Consider the physical and chemical properties of the most important carboxylic acids. 6. Find out the areas of application of some carboxylic acids.

All carboxylic acids have a functional group Carbonyl group O - C Hydroxyl group OH Carboxyl group General formula R C O OH Or for saturated monobasic acids O OH Cn H2n+1C? What are carboxylic acids called? Carboxylic acids are organic compounds whose molecules contain a carboxyl group, COOH, bonded to a hydrocarbon radical. ? How are carboxylic acids genetically related to the previously studied class of aldehydes? R C O H + [O] R C O OH [O]= KMnO4, K2Cr2O7+ H2SO4 conc. Carboxylic acids are organic compounds whose molecules contain a carboxyl group, COOH, bonded to a hydrocarbon radical. R C

Classification of carboxylic acids Depending on the number of carboxyl groups Monobasic Dibasic (acetic) (oxalic) O OH CH3C C - CO OH O HO Depending on the nature of the radical Saturated (propionic) O CH3- CH2-C OH Unsaturated (acrylic) O CH2= CH-C OH Polybasic (lemon) O H2C – C OH HC - C O OH O H2C - C OH Aromatic (benzoic) C O OH According to the content of C atoms: C1C9 lower, C10 and higher

Classify the proposed acids 1) CH3 – CH2 – CH2 – CH2 COOH 1. Monobasic, saturated, lower 2) HOOC CH2 CH2 COOH 2. Dibasic, saturated, lower 3) COOH CH3 3. Monobasic, saturated, lower 4) CH3 – (CH2 )7 –CH = CH (CH2)7 COOH 4. Monobasic, unsaturated, higher 5) HOOC –CH2 CH – CH2 COOH COOH 5. Polybasic, saturated, lower

NOMENCLATURE OF CARBOXYLIC ACIDS NOMENCLATURE OF CARBOXYLIC ACIDS ALKANE ACID + OB + ANIC ACID ALKANE METHANIC ACID METHANE ACID (FOMIC ACID)

2 1 CH3 – COOH COOH CH3 – ETHANETHANIC ACID ACID (ACETIC ACID)) 33 44 11 COOH CH3 – CH2 – CH2 COOH CH3 – CH2 – CH2 22 BUTANIC ACID BUTANIC ACID (BUTICAL ACID) (BUTICAL ACID LOT)

44 22 55 11 COOH CH3 – CH2 – CH2 – CH2 – COOH CH3 – CH2 – CH2 – CH2 – 33 PENTANIC ACID PENTANE (VALERIAN ACID) (VALERIAN ACID) 22 NOOS – NOOS 11 COOH – COOH ETHANETHANEDIDI OVAIC ACID (OXALIC ACID) (OXALIC ACID)

Homologous series of carboxylic acids Homologous series of carboxylic acids Chemical formula Systematic name of the acid Trivial name of the acid Name of the acidic residue Formate Acetate Propionate Butyrate capronate capricate HCOOH CH3COOH CH3CH2COOH CH3CH2CH2COOH CH3CH2CH2CH2COOH CH3(CH2)4–COOH Methane Ethanic Propane Butane Pen tanovaya hexane formic acetic acid propionic oil nylon valerian valerinate CH3(CH2)8 – COOH Decane capric CH3(CH2)14 – COOH CH3(CH2)16 COOH Hexadecane Octadecane Palmitic palmitate Stearic stearate

Algorithm for naming carboxylic acids: 1. Find the main chain of carbon atoms and number it, starting with the carboxyl group. 2. We indicate the position of the substituents and their name(s). 3. After the root, indicating the number of carbon atoms in the chain, comes the suffix “oic” acid. 4. If there are several carboxyl groups, then a numeral is placed before “ova” (di, three...) Example: 4 CH3 3 CH 2 CH2 1 COOH CH3 3 methylbutane + ova = 3methylbutanoic acid acid

GIVE A NAME TO THE SUBSTANCES GIVE A NAME TO THE SUBSTANCES ACCORDING TO THE INTERNATIONAL NOMENCLATURE (2 – METHYLPROPANE (2 – METHYL PROPANOIC ACID) ACID) CH3 – CH – COOH 1.1. CH3 – CH – COOH CH3CH3 2. CH3 – CH2 – CH – CH – COOH 2. CH3 – CH2 – CH – CH – COOH CH3CH3 CH3CH3 (2, 3 – DIMETHYL PENTA PENTA (2, 3 – DIMETHYL NNOVAIC ACID) ACID) 3. CH3 – CH = CH – CH – COOH 3. CH3 – CH = CH – CH – COOH CH3CH3 4. HOOC – CH2 – CH – COOH 4. HOOC – CH2 – CH – COOH CC22NNH55 (2 – METHYLPENT (2 – METHYL ACID) ACID) PENTENENE – 3– 3 – OVA – OVA (2 – ETHYL BUTANE (2 – ETHYL BUTANEDIIOIC ACID) ACID)

Algorithm for writing formulas of carboxylic acids: 1. Select the root of the word based on which write down the carbon skeleton in the composition, which includes a carboxyl group. 2. We number the carbon atoms, starting with the carboxyl group. 3. We indicate substituents according to numbering. 4. It is necessary to add the missing hydrogen atoms (carbon is tetravalent). 5. Check that the formula is written correctly. Example: 4 3 2 1 C C C COOH 2methylbutanoic acid. 4 3 2 1 C C C COOH 4 3 2 1 CH3 CH2 CH COOH CH3 CH3

PHYSICAL PROPERTIES PHYSICAL PROPERTIES CC11 – – CC33 Liquids with a characteristic Liquids with a characteristic pungent odor, pungent odor, highly soluble in water highly soluble in water CC44 – C – C99 Viscous oily Viscous oily liquids with liquids with an unpleasant odor, poorly unpleasant odor, poorly soluble soluble in water in water CC1010 and more more Solids, Solids, odorless, odorless, insoluble insoluble in water in water

Physical properties of acetic acid: Physical state liquid Color colorless transparent liquid Odor pungent acetic acid Solubility in water is good Boiling point 118 º C Melting point 17 º C

Dependence of the physical properties of carboxylic acids on the structure of the molecule: Lower carboxylic acids are liquids; higher - solid substances. The greater the relative molecular weight of the acid, the less its odor. As the relative molecular weight of the acid increases, solubility decreases. The homologous series of aldehydes begins with two gaseous substances (at room temperature), and there are no gases among carboxylic acids. What is this connected with?

Chemical properties of carboxylic acids Chemical properties of carboxylic acids I. Common with inorganic acids dissociate: 1. Soluble carboxylic acids in aqueous solutions CH3 – COOH CH3 – COO + H + Is the medium acidic? How will the color of indicators change in an acidic environment? 2. Interaction with metals in the electrochemical voltage series up to hydrogen: Litmus (violet) - turns red Methyl orange - turns pink Phenophthalein - does not change color 2CH3 - COOH + Mg Acetic acid 2CH3 COOH + Zn Acetic acid (CH3 -COO) 2Mg Magnesium acetate ( CH3 – COO)2Zn Zinc acetate + H2 + H2 When a metal reacts with a carboxylic acid solution, hydrogen and salt I are formed. Common with inorganic acids

3. Interaction with basic oxides: 2 CH3 – COOH + CuO Acetic acid (CH3 – COO) 2Сu Copper acetate t + H2O 4. Interaction with metal hydroxides (neutralization reaction) CH3 – COOH + HO –Na Acetic acid 2CH3 – COOH + Cu (OH)2 Acetic acid CH3COONa Sodium acetate + H2O (CH3COO)2Cu Copper acetate + H2O 5. Interaction with salts of weaker and more volatile acids (eg, carbonic, silicic, hydrogen sulfide, stearic, palmitic...) 2CH3– COOH Acetic acid + Na2CO3 sodium carbonate 2CH3COONa Sodium acetate + H2CO3 CO2 H2O

Specific properties of formic acid “Silver Mirror” reaction O + Ag2O t 2Ag + H2CO3 H C OH Formic acid Silver oxide silver CO2 H2O

Occurrence in nature and use of carboxylic acids Formic acid (Methanoic acid) - Chemical formula CH2O2, or HCOOH. Formic acid was discovered in the acidic secretions of red ants in 1670 by the English naturalist John Ray. Formic acid is also present in the finest nettle hairs, in bee venom, pine needles, and is found in small quantities in various fruits, tissues, organs, and animal and human secretions.


Question: Why can’t you wet the area of an ant bite or nettle sting with water? This only leads to increased pain. Why does the pain subside if the wounded area is moistened with ammonia? What else can be used in this case? When formic acid is dissolved in water, a process of electrolytic dissociation occurs: HCOOH HCOO + H As a result, the acidity of the environment increases, and the process of skin corrosion intensifies. In order for the pain to subside, you need to neutralize the acid, for which you need to use solutions that have an alkaline reaction, such as a solution of ammonia. HCOOH + NH4OH HCOONH4 + H2O or HCOOH + NaHCO3 HCOONa + CO2 + H2O

Acetic acid (ethanoic acid) This is the first acid obtained and used by man. “Born” more than 4 thousand years ago in Ancient Egypt. At the turn of the 17th – 18th centuries in Russia it was called “sour moisture.” The first time we got it was during the souring of wine. The Latin name is Acetum acidum, hence the name of the salts - acetates. Glacial acetic acid at temperatures below 16.8 ºС hardens and becomes like ice. Acetic essence is a 70% acid solution. Table vinegar 6% or 9% acid solution. Acetic acid is found in animal secretions (urine, bile, feces), plants (particularly green leaves), sour milk and cheese; formed during fermentation, rotting, souring of wine and beer, and during the oxidation of many organic substances;

Application of acetic acid Application of acetic acid Aqueous solutions of acetic acid are widely used in the food industry (food additive E 260) and household cooking, as well as in canning; in the production of: drugs, HP, aspirin; artificial fibers, eg, silk acetate; indigo dyes, non-flammable film, organic glass; varnish solvents; chemical plant protection products, plant growth stimulants; Sodium acetate CH3COONa is used as a preservative for blood intended for transfusion; Potassium acetate CH3COOK – as a diuretic; Lead acetate (CH3COO)2 Pb for determination of sugar in urine; Acetates of iron (III) (CH3COO)3Fe, aluminum (CH3COO)3Al and chromium (III) (CH3COO)3Cr are used in the textile industry for mordant dyeing; Copper (II) acetate (CH3COO)2Cu is included in the preparation for controlling plant pests, the so-called Parisian green; Aqueous solutions of acetic acid are widely used in the food industry (food additive E 260) and household cooking, as well as in canning; in the production of: drugs, HP, aspirin; artificial fibers, eg, acetate silk; indigo dyes, non-flammable film, plexiglass; varnish solvents; chemical plant protection products, plant growth stimulants; Sodium acetate CH3COONa is used as a preservative for blood intended for transfusion; Potassium acetate CH3COOK - as a diuretic; Acetate lead (CH3COO)2 Pb for determining sugar in urine; Acetates of iron (III) (CH3COO)3Fe, aluminum (CH3COO)3Al and chromium (III) (CH3COO)3Cr are used in the textile industry for mordant dyeing; Copper acetate (II) ( CH3COO)2Cu is part of a preparation for controlling plant pests, the so-called Parisian green;

Use of acetic acid The use of alcohol vinegar in cosmetology is known. Namely, to give softness and shine to hair after perm and permanent coloring. To do this, it is recommended to rinse your hair with warm water with the addition of alcohol vinegar (34 tablespoons of vinegar per 1 liter of water). In folk medicine, vinegar is used as a nonspecific antipyretic. for headaches using lotions. for insect bites using compresses. Did you know that if you need to unscrew a rusty nut, it is recommended to put a rag soaked in acetic acid on it in the evening? It will be much easier to unscrew this nut in the morning. During the day, 400 g of acetic acid is formed in the body? This would be enough to make 8 liters of ordinary vinegar, indispensable in the production of perfume products

N N S C 3 O O Of all the acids, It is, of course, the prima. Present everywhere, both visibly and invisibly. It is present in animals and plants, Technology and medicine are with it forever. Its offspring are acetates. Very necessary “guys”. The well-known aspirin, like a good gentleman, reduces the patient’s fever and restores health. This is copper acetate. He is a friend and brother to the plants, Kills their enemies. Acid still has some use - It dresses us in acetate silk. And whoever loves dumplings has known vinegar for a long time. There is also the question of cinema: Well, everyone needs to know that without acetate film we cannot see cinema. Of course there are other uses. And you know them without a doubt. But the main thing that is said, friends, is “Bread of the industry”: acetic acid. Aqueous solution of copper acetate CH3COOH
Conclusions 1.Carboxylic acids are organic compounds whose molecules contain a carboxyl group - COOH, associated with a hydrocarbon radical. 2.Carboxylic acids are classified: by basicity (one, two and polybasic) by hydrocarbon radical (saturated, unsaturated and aromatic) by the content of C atoms (lower and higher) 3. The name of a carboxylic acid is composed of the name of an alkane + ovalic acid. 4. As the molecular weight of a carboxylic acid increases, the solubility and strength of the acid decreases. 5. Like inorganic acids, soluble carboxylic acids dissociate in an aqueous solution, forming hydrogen ions and changing the color of the indicator. They react with metals (up to H), basic and amphoteric oxides and hydroxides, and salts of weaker acids, forming salts. 6 Widely distributed in nature and have great practical importance for humans.

Homework 1. 2. §14, exercises No. 6 exercise No. 9 Queen Cleopatra, on the advice of the court physician, dissolved the largest pearl known to jewelers in vinegar, and then took the resulting solution for some time. What reaction did Cleopatra make? What connection was she taking? 3. Prepare a report on higher carboxylic acids

Carboxylic acids
Slides: 41 Words: 1295 Sounds: 24 Effects: 62Carboxylic acids. All carboxylic acids have a functional group. What are carboxylic acids called? Self-control task. Nomenclature of carboxylic acids. Carboxylic acids in nature. General formula of carboxylic acids. The structure of the carboxyl group. Physical properties of carboxylic acids. Physical properties of saturated carboxylic acids. Chemical properties of carboxylic acids. Reaction with halogens. Preparation of carboxylic acids. Carboxylic acid molecules form dimers. Repeat the determination of carboxylic acids. Nomenclature of esters. Esters in nature. Copper acetate. - Acids 1.ppt
Interaction of carboxylic acids
Slides: 14 Words: 359 Sounds: 0 Effects: 0Carboxylic acids. The composition of saturated monobasic carboxylic acids corresponds to the general formula O R – C OH. Classification. Examples of acids. Isomerism. Structure. Physical and chemical properties. Lower carboxylic acids are liquids with a pungent odor, highly soluble in water. CH3COOH + SOCl2 CH3COCl + HCl + SO2. Methods of obtaining. 4. Hydrolysis of esters: 5. Hydrolysis of acid anhydrides: Specific methods of preparation. For individual acids, there are specific methods of preparation: The use of carboxylic acids. Glue. Herbicides. Preservative, seasoning. Perfumes, cosmetics. - Carboxylic acids.ppt
Properties of carboxylic acids
Slides: 21 Words: 424 Sounds: 0 Effects: 96Carboxylic acids. Organic chemistry 11th grade. –COOH Carboxyl group. Methanedioic acid (dicarboxylic acid). 2-hydroxypropanetric acid (2-hydroxy-1,2,3-propanetricarboxylic acid). Physical properties. Polarization of a molecule. Possibility of forming hydrogen bonds. High boiling points. Dimer of acetic acid. Solubility in water. The structure of the carboxyl group. Radicals. Nucleophiles. Electrophiles. Chemical properties. They exhibit general properties of acids. Carboxylic acid. Magnesium carboxylate. Ethanoic acid. Magnesium ethanate. Calcium ethate. Sodium methane. - Carboxylic acids 1.ppt
Carboxylic acids in nature
Slides: 20 Words: 379 Sounds: 0 Effects: 0Carboxylic acids. Formulas and names of some common acids. Formulas and names of acid residues. Lower carboxylic acids. - colorless liquids with a pungent odor. As the molar mass increases, the boiling point increases. ...Carboxylic acids in nature: C2H5COOH – tree resin. C3H7COOH – butter. C4H9COOH – roots of valerian herb. C6H5COOH – clove oil. The simplest carboxylic acids. Note that... the acid HCOOH enters into a “silver mirror” reaction like aldehydes: And decomposes under the influence of water-removing agents: Obtaining carboxylic acids. - Carboxylic acids 2.ppt
Carboxylic acid
Slides: 9 Words: 193 Sounds: 0 Effects: 0Presentation. Carboxylic acids. The purpose of the lesson. Consider the basics of international and trivial nomenclatures, the use of carboxylic acids. Analyze the structure of the carboxyl group and predict the chemical behavior of carboxylic acids. Classification of carboxylic acids. According to the number of carboxyl groups. Unlimited. Aromatic. Monobasic. Dibasic. Polybasic. Limit. According to the type of hydrocarbon radical. General formula of monobasic acids of the limiting series. CnH2n+1COOH where n can be zero. The simplest carboxylic acids. Formulas and names of some carboxylic acids. - Carboxylic acids 3.ppt
Carbonic acids
Slides: 14 Words: 889 Sounds: 0 Effects: 0Carboxylic acids. Structure. Organic acids containing one carboxyl group in the molecule are monobasic. Carboxylic acids containing two carboxyl groups are called dibasic. Oxalic acid. Saturated (or saturated) carboxylic acids do not contain α-bonds in the hydrocarbon radical. For example, benzoic acid: Nomenclature and isomerism. The numbering of carbon atoms begins with the carboxyl group. Methane (formic) acid. Ethanedionic (oxalic) acid. Physical properties of saturated monobasic carboxylic acids. Chemical properties. - Carboxylic acids 4.ppt
Unsaturated carboxylic acids
Slides: 11 Words: 305 Sounds: 0 Effects: 37Unsaturated carboxylic acids. Physical properties. Isomerism. Methods of obtaining. Chemical properties. Biological structure. Application. Acrylic acid. Methacrylic acid. CH2=CH-coon. CH2=c-coon. CH3. Oleic acid. CH3 – (CH2)7-CH=CH-(CH2)7 -coon. Linoleic acid. CH3-(CH2)4-CH=CH-CH2-CH=CH-(CH2)7-coon. Linolenic acid. CH3-SN2-CH=CH-CH2-CH=CH-CH2-CH=CH-(CH2)7-coon. Isomerism of unsaturated carboxylic acids. Carbon chain. Double bond positions. Geometric (cis-trans isomerism). Geometric isomers of oleic acid. Nitrogen oxides. Elaidic acid. - Carboxylic acids 5.ppt
Acetic and stearic acids
Slides: 14 Words: 236 Sounds: 0 Effects: 0Grade 9 Lesson 6. Carboxylic acids. Lesson plan. The concept of carboxylic acids. Acetic acid. Stearic acid. Questions and exercises. 1. The concept of carboxylic acids. The structural formula of the carboxyl group is: O C O H. -Coon. Carboxylic acid. Carboxylate anion. Among carboxylic acids, there are a ton of famous “persons”. In acids there are carboxyl groups, but all the acids here are not strong. 2. Acetic acid. Acetic acid CH3COOH is the oldest of organic acids. Pure acetic acid is a non-ferrous liquid with a pungent odor. Acetic acid is used in large quantities in the chemical industry. - Carboxylic acids 6.ppt
Carboxylic acids chemistry
Slides: 14 Words: 341 Sounds: 0 Effects: 0Carboxylic acids. Lecture. 1. Determination of acids. 2. Homologous series. The simplest representatives are saturated monobasic carboxylic acids. Carbonyl and hydroxyl groups, which form a single functional carboxyl group. The properties of carboxylic acids depend on availability. Substitution occurs at the 2nd carbon atom and occurs on the catalyst. This reaction is intermediate for the production of amino acids. Carboxylic group of acids. Aldehyde group. Oleic acid. 9,10 – dibromostearic acid. Oleic acid belongs to the higher unsaturated carboxylic acids. - Carboxylic acids chemistry.ppt
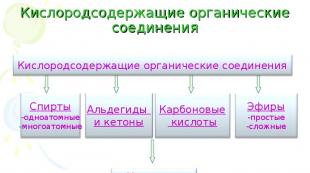
Classes of carboxylic acids
Slides: 33 Words: 809 Sounds: 0 Effects: 13Oxygen-containing organic compounds. Carboxylic acids. Preparation of carboxylic acids. General formula. Definition. Classification of carboxylic acids. Classification of carboxylic acids according to the nature of the hydrocarbon radical. Classification of carboxylic acids by the number of functional groups. Representatives of monobasic carboxylic acids. Homologous series of acids. Nomenclature of carboxylic acids. Isomerism of carboxylic acids. Physical properties of carboxylic acids. Application of carboxylic acids. Production of organic compounds. Methylbutanoic acid. Functional group. - Classes of carboxylic acids.ppt
Examples of carboxylic acids
Slides: 15 Words: 563 Sounds: 0 Effects: 10Carboxylic acids. Study the structure. These are organic substances. Valeric acid. Indicator. Formic acid. Classification of carboxylic acids. Oxalic acid. Lemon acid. Acetic acid. Chemical properties of carboxylic acids. They form ethers. Stearic acid. Acids. - Examples of carboxylic acids.ppt
Properties of carboxylic acids
Slides: 24 Words: 328 Sounds: 0 Effects: 0Development of a lesson in chemistry. The concept of a functional group. Maybe the one who thinks he can. Characterize a new class of organic substances. Acid. Acidic properties. Carboxyl group. Monobasic carboxylic acids. Organic substances. Characterization plan. Carboxylic acids. Classification. Structural isomerism. Physical properties. Dimer structure. Electron shift. Chemical properties. Dissociation in aqueous solution. Properties. - Properties of carboxylic acids.ppt
Carboxylic acids and their properties
Slides: 28 Words: 1463 Sounds: 0 Effects: 65Carboxylic acids. Discovery of acids. Interesting historical facts related to organic acids. Nettle containing formic acid. Carboxylic acids in nature. Formic acid was first isolated in the 17th century. Acetic acid is widely distributed in nature. Organic substances. R-COOH. Nomenclature of carboxylic acids. Classification. Dicarboxylic acids. Algorithm for naming carboxylic acids. Algorithm for writing formulas of carboxylic acids. Name the substance. Ethanoic or acetic acid. Chloroacetic acid or chloroethanoic acid. Which acid is stronger? - Carboxylic acids and their properties.pptx
Chemical properties of carboxylic acids
Slides: 17 Words: 666 Sounds: 0 Effects: 25Chemical properties of carboxylic acids. The road to knowledge. Chemical properties. Functional group. Formulas of carboxylic acids. Name of carboxylic acids. Trivial names for carboxylic acids. The structure of the carboxyl group. Formic acid. Salicylic acid. Oxalic acid. Chemical properties of inorganic acids. General properties of carboxylic acids. Carboxylic acids. Task. - Chemical properties of carboxylic acids.ppt
Carboxylic acid derivatives
Slides: 19 Words: 412 Sounds: 0 Effects: 127Functional derivatives of carboxylic acids. Carboxyl. Ketone. Substitution products for carboxylic acids. Esters. Esterification. Methane (formic) acid. Amides. Metanamide. Chemical properties of esters and amides. Acid hydrolysis. Reversible process. Catalyst. Hydrolysis of amides. Leaving nucleophile. Hydrolysis of esters. Esters of mineral acids. Esters of sulfuric acid. Esters of phosphoric acid. - Derivatives of carboxylic acids.ppt
Saturated carboxylic acids
Slides: 41 Words: 1517 Sounds: 24 Effects: 62Structure of monobasic carboxylic acids. Carboxylic acids. All carboxylic acids have a functional group. Self-control task. Trivial names. Homologous series. Physical properties. Chemical properties of carboxylic acids. React with basic oxides. Which of these acids is stronger? Preparation of carboxylic acids. Ethane. Definition. Organic substances. Select the carboxylic acid formula. Repeat the determination of carboxylic acids. Theory. Nomenclature of esters. Isobutyl acetate. Name the carboxylic acids. Dimethylhexanoic acid. Copper acetate. - Saturated carboxylic acids.ppt
Saturated monobasic carboxylic acids
Slides: 13 Words: 764 Sounds: 1 Effects: 20Structure and nomenclature of saturated carboxylic acids. Saturated monobasic carboxylic acids. Carboxylic acids. Types of isomerism. Carbon atom. Name the carboxylic acids. Trivial names. Monobasic carboxylic acids. Physical properties. History of discovery. Formic acid. Gaseous substances. Organic substances. - Limit monobasic carboxylic acids.ppt
Fatty acid
Slides: 44 Words: 2065 Sounds: 0 Effects: 0Polyunsaturated fatty acids as signaling molecules. 1. Lipidomics and lipidology. Classical paradigm: Paradigm of the postgenomic era: Interest in lipidomics is associated with: 1. Changes in general approaches to the study of cells. Lipidology. Tissues and cells. Lipid extracts. Lipid profile. Enzymes, proteins. System management. Lipidomics. The place of lipidomics among other “-omics”. Construction of the system. Prediction. Diagnostics. Treatment. 2. Arachidonic acid and other polyene fatty acids as signaling molecules. n-6 Linoleic (18:2n-6) g-Linolenic (18:3n-6) Dihomo-g - linolenic (18:3n-6) Arachidonic (20:4n-6) Docosatetraenoic (22:4n-6) Docosapentaenoic (22:5n-6). - Fatty acids.ppt
Omega acid
Slides: 12 Words: 617 Sounds: 0 Effects: 0OMEGA 3 and OMEGA 6 polyunsaturated fatty acids (PUFAs). The structure and function of the epithelium of the mucous membranes and skin is disrupted. Skin damage is also typical: dermatological disorders occur, and there is a predisposition to infection with subcutaneous mites. Eicosapentaenoic and docosahexaenoic fatty acids, belonging to the OMEGA 3 group, slow down the growth of tumors. With insufficient intake of PUFAs, animals experience slower growth, decreased fertility, and deterioration in physiological condition. Recently, studies have been conducted showing the effect of OMEGA 3 and OMEGA 6 fatty acids as modulators of the immune system. -