Quantum field theory. Quantum physics for dummies: the essence in simple words. Even a child will understand. More precisely, especially a child! Quantum theory says that
The demonstration that disproved the great Isaac Newton's assumptions about the nature of light was stunningly simple. This “might be easily repeated wherever the sun shines,” English physicist Thomas Young told members of the Royal Society in London in November 1803, describing what is now known as the double-slit experiment, or Young’s experiment. Jung did not look for difficult paths and did not make a buffoon show out of his experience. He simply came up with an elegant and decisive experiment to demonstrate the wave nature of light using ordinary materials at hand, thereby disproving Newton's theory that light was made of corpuscles or particles.
Jung's experience.
Young's experiment (double slit experiment)- an experiment conducted by Thomas Young and which became experimental proof of the wave theory of light.
In the experiment, a beam of monochromatic light is directed onto an opaque screen screen with two parallel slits, behind which a projection screen is installed. The width of the slits is approximately equal to the wavelength of the emitted light. The projection screen produces a series of alternating interference fringes. The interference of light proves the validity of the wave theory.
But the birth of quantum physics in the early 1900s made it clear that light is made of tiny, indivisible units or quanta of energy that we call photons. Young's experiment, which demonstrated single photons or even individual particles of matter such as electrons and neutrons, forced humanity to think about the nature of reality itself. Some have even used this experiment to argue that the quantum world is influenced by human consciousness, giving minds food for thought about our place in the ontology of the Universe. But can a simple experiment really cause such a change in the worldview of everyone?
The dubious concept of measurement
In a modern interpretation of the experience, a beam of monochromatic light is directed onto an opaque screen screen with two parallel slits, behind which a projection screen is installed. It registers the impact of particles passing through the slots. In the case of photons, this is a photographic plate. Logically, one would expect that photons should pass through one slit or another and accumulate behind them.
But that's not true. They go to certain parts of the screen and simply avoid others, creating alternating bands of light and dark - so-called interference fringes. They are produced when two sets of waves overlap each other. Where the waves are in the same phase, the amplitude will add up and result in amplifying interference - light stripes. When the waves are out of phase, weakening interference occurs - dark fringes.
But there is only one photon that will pass through both slits. It's like a photon passing through both slits at once and interfering with itself. This doesn't fit into the classic picture.
From a mathematical point of view, a photon passing through both slits is not a physical particle or a physical wave, but something called a wave function - an abstract mathematical function that represents the state of the photon (in this case, its position). The wave function behaves like a wave. It hits both slits and new waves emanate from each, spreading and eventually colliding with each other. The combined wave function can be used to calculate the probability of where the photon will be located.

Jacob Biamonte, Skoltech, - about what quantum computers can do right now
The photon is very likely to be where the two wave functions create increasing interference, and is unlikely to be in regions of weakening interference. The measurement - in this case the interaction of the wave function with the photographic plate - is called the "collapse" of the wave function or von Neumann reduction. This process occurs during a measurement in one of those places where the photon materializes.
Von Neumann reduction (wave function reduction or collapse)- an instantaneous change in the description of the quantum state (wave function) of an object that occurs during measurement. Since this process is essentially non-local, and the instantaneity of the change implies the propagation of interactions faster than the speed of light, it is believed that it is not a physical process, but a mathematical method of description.
There is nothing that man does not observe
This seemingly strange collapse of the wave function is the source of many difficulties in quantum mechanics. Before the passage of light, it is impossible to say with certainty where a single photon will end up. It can appear anywhere with non-zero probability. It is impossible to draw the path of a photon from a source to a point on the screen. The trajectory of a photon cannot be predicted; it is not like an airplane flying the same route from San Francisco to New York.
Werner Heisenberg, like other scientists, postulated that reality, from a mathematical point of view, does not exist until there is an observer.
“The idea of an objective real world, whose parts exist just like rocks or trees, and independent of whether we observe them or not, is impossible,” he wrote. John Wheeler also used a version of the double-slit experiment to argue that “no elementary quantum phenomenon is truly a quantum phenomenon until it is witnessed by others (“observable”).
Werner Carl Heisenberg is the author of a number of fundamental works in quantum theory: he laid the foundations of matrix mechanics, formulated the uncertainty relation, applied the formalism of quantum mechanics to the problems of ferromagnetism, the anomalous Zeeman effect, and others.
Subsequently, he actively participated in the development of quantum electrodynamics (Heisenberg-Pauli theory) and quantum field theory (S-matrix theory), and in the last decades of his life he made attempts to create a unified field theory. Heisenberg owns one of the first quantum mechanical theories of nuclear forces. During World War II he was the leading theoretician of the German nuclear project.
John Archibald Wheeler introduced several terms (quantum foam, neutron moderation), including two that subsequently became widespread in science and science fiction - black hole and wormhole.
But quantum theory does not at all articulate what a “measurement” should be. It simply postulates that the measuring device must be classical, without defining where the fine line is between classical and false measurement. This gives rise to the emergence of supporters of the idea that human consciousness causes the collapse of the wave function. In May 2018, Henry Stapp and his colleagues argued that the double-slit experiment and its modern variants suggest that “the conscious observer may be indispensable” for making sense of quantum theory and the idea that the mind of each person underlies the material world.
But these experiments are not empirical evidence. In the double slit experiment, all you can do is calculate the probability. If the probability appears in tens of thousands of identical photons in an experiment, it can be argued that the wave function is collapsing - thanks to a dubious process called measurement. That's all that can be done.
Regardless of the person
Additionally, there are other ways to interpret Young's experiment. For example, the de Broglie-Bohm theory, which states that reality is both a wave and a particle. And the photon is always directed to the double slit with a certain initial position and passes through one slit or the other. Therefore, each photon has a trajectory. This is called propagation of the pilot wave, which passes through both slits, interference occurs, and then the pilot wave directs the photon into the region of amplifying interference.

Bohmian trajectories for an electron passing through two slits. A similar picture was also extrapolated from weak measurements of single photons.Image: thequantumphysics
In addition to the wave function in the space of all possible configurations, the de Broglie-Bohm theory postulates a real configuration that exists without even being measured. In it, the wave function is defined for both slits, but each particle has a well-defined trajectory that passes through exactly one slit. The final position of the particle on the detector screen and the slit through which it passes is determined by the initial position of the particle. Such an initial position is unknowable or uncontrollable on the part of the experimenter, so that there is an appearance of randomness in the detection pattern.
In 1979, Chris Dewdney and his colleagues at Birbeck College modeled theoretical trajectories of particles passing through two slits. In the last decade, experimenters have become convinced that such trajectories exist, albeit using a rather controversial method called weak measurement. Despite the contradictions, experiments show that the de Broglie-Bohm theory explains the behavior of the quantum world.
Birkbeck (University of London)- a research and educational institution with evening courses, specializing in the provision of higher education. It is part of the University of London.
The essential thing about these measurements is that the theory does not need observers, measurements or human participation.
So-called collapse theories claim that the collapse of wave functions occurs randomly. The more particles there are in a quantum system, the more likely it is. Observers simply record the result. Markus Arndt's team at the University of Vienna tested these theories by sending larger and larger particles through slits. Collapse theories state that when particles of matter become more massive than a certain amount, they cannot remain in the quantum field passing through both slits at the same time, this will destroy the interference pattern. Arndt's team sent a particle with more than 800 atoms through the slits, and a redistribution of light intensity did occur. The search for the critical value continues.
Roger Penrose has his own version of collapse theory: the higher the mass of an object in a quantum field, the faster it will change from one state to another due to gravitational instability. Again, this is a theory that does not require human intervention. Consciousness has nothing to do with it. Dirk Bouwmeester at the University of California, Santa Barbara, tests Penrose's idea with Young's experiment.
Essentially, the idea is to not just force a photon to pass through both slits, but to force one of the slits into a superposition - in two places at the same time. According to Penrose, the displaced slit will either remain in superposition or collapse while the photon passes, leading to different types of interference patterns. The collapse will depend on the size of the cracks. Bouwmeester has been working on this experiment for a decade and will soon be able to confirm or refute Penrose's claims.

Quantum computer will reveal the mysteries of genetics
Unless something revolutionary happens, these experiments will show that we cannot yet claim absolute knowledge of the nature of reality. Even if the attempts are motivated mathematically or philosophically. And the conclusions of neuroscientists and philosophers who disagree with the nature of quantum theory and claim that the collapse of wave functions takes place are at best premature, and at worst erroneous and only mislead everyone.
Physics gives us an objective understanding of the world around us, and its laws are absolute and apply to all people without exception, regardless of social status and persons.
But such an understanding of this science was not always present. At the end of the 19th century, the first untenable steps were taken towards creating a theory of black physical body radiation based on the laws of classical physics. From the laws of this theory it followed that a substance must emit certain electromagnetic waves at any temperature, reduce the amplitude to absolute zero and lose its properties. In other words, thermal equilibrium between radiation and a specific element was impossible. However, such a statement was in conflict with real everyday experience.
Quantum physics can be explained in more detail and understandably as follows. There is a definition of an absolutely black body, which is capable of absorbing electromagnetic radiation of any wave spectrum. The length of its radiation is determined only by its temperature. In nature there cannot be absolutely black bodies that correspond to an opaque closed substance with a hole. When heated, any piece of an element begins to glow, and with a further increase in the degree it turns red, and then white. Color practically does not depend on the properties of the substance; for an absolutely black body it is characterized solely by its temperature.
Note 1
The next stage in the development of the quantum concept was the teaching of A. Einstein, which is known under the Planck hypothesis.
This theory enabled the scientist to explain all the laws of the unique photoelectric effect that do not fit within the limits of classical physics. The essence of this process is the disappearance of matter under the influence of fast electrons of electromagnetic radiation. The energy of the emitted elements does not depend on the coefficient of absorbed radiation and is determined by its characteristics. However, the number of emitted electrons depends on the saturation of the rays
Repeated experiments soon confirmed Einstein's teachings, not only with the photoelectric effect and light, but also with x-rays and gamma rays. The A. Compton effect, which was discovered in 1923, presented to the public new facts about the existence of certain photons through the arrangement of elastic scattering of electromagnetic radiation on free, small electrons, accompanied by an increase in the range and wavelength.
Quantum field theory
This doctrine allows us to determine the process of introducing quantum systems into a framework called degrees of freedom in science, which assume a certain number of independent coordinates, which are extremely important for indicating the overall movement of a mechanical concept.
In simple words, these indicators are the main characteristics of movement. It is worth noting that interesting discoveries in the field of harmonious interaction of elementary particles were made by researcher Steven Weinberg, who discovered the neutral current, namely the principle of the relationship between leptons and quarks. For his discovery in 1979, the physicist became a Nobel Prize laureate.
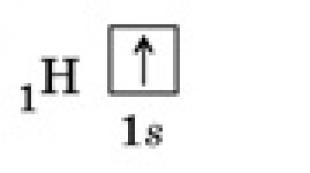
In quantum theory, an atom consists of a nucleus and a specific cloud of electrons. The basis of this element includes almost the entire mass of the atom itself - more than 95 percent. The nucleus has an exclusively positive charge, defining the chemical element of which the atom itself is a part. The most unusual thing about the structure of the atom is that the nucleus, although it makes up almost all of its mass, contains only one ten-thousandth of its volume. It follows from this that there is indeed very little dense matter in an atom, and the rest of the space is occupied by an electron cloud.
Interpretations of quantum theory - the principle of complementarity
The rapid development of quantum theory has led to a radical change in classical ideas about such elements:
- structure of matter;
- movement of elementary particles;
- causality;
- space;
- time;
- the nature of cognition.
Such changes in people's consciousness contributed to a radical transformation of the picture of the world into a clearer concept. The classical interpretation of a material particle was characterized by a sudden release from the environment, the presence of its own movement and a specific location in space.
In quantum theory, an elementary particle began to be represented as the most important part of the system in which it was included, but at the same time it did not have its own coordinates and momentum. In the classical cognition of movement, the transfer of elements that remained identical to themselves along a pre-planned trajectory was proposed.
The ambiguous nature of particle division necessitated the abandonment of such a vision of motion. Classical determinism gave way to the leading position to the statistical direction. If previously the entire whole in an element was perceived as the total number of component parts, then quantum theory determined the dependence of the individual properties of the atom on the system.
The classical understanding of the intellectual process was directly related to the understanding of a material object as fully existing in itself.
Quantum theory has demonstrated:
- dependence of knowledge about the object;
- independence of research procedures;
- completeness of actions on a number of hypotheses.
Note 2
The meaning of these concepts was initially far from clear, and therefore the main provisions of quantum theory have always received different interpretations, as well as various interpretations.
Quantum statistics
In parallel with the development of quantum and wave mechanics, other components of quantum theory were rapidly developing - statistics and statistical physics of quantum systems, which included a huge number of particles. On the basis of classical methods of movement of specific elements, a theory of the behavior of their integrity was created - classical statistics.
In quantum statistics there is absolutely no possibility of distinguishing between two particles of the same nature, since the two states of this unstable concept differ from each other only by the rearrangement of particles of identical power of influence on the principle of identity itself. This is how quantum systems mainly differ from classical scientific systems.
An important result in the discovery of quantum statistics is the proposition that each particle that is part of any system is not identical to the same element. This implies the importance of the task of determining the specifics of a material object in a specific segment of systems.
The difference between quantum physics and classical
So, the gradual departure of quantum physics from classical physics consists in the refusal to explain individual events occurring in time and space, and the use of the statistical method with its probability waves.
Note 3
The goal of classical physics is to describe individual objects in a certain sphere and formulate laws governing the change of these objects over time.
Quantum physics occupies a special place in science in the global understanding of physical ideas. Among the most memorable creations of the human mind is the theory of relativity - general and special, which is a completely new concept of directions that combines electrodynamics, mechanics and the theory of gravity.
Quantum theory was able to finally break ties with classical traditions, creating a new, universal language and an unusual style of thinking, allowing scientists to penetrate the microworld with its energetic components and give its complete description by introducing specifics that were absent in classical physics. All these methods ultimately made it possible to understand in more detail the essence of all atomic processes, and at the same time, it was this theory that introduced an element of randomness and unpredictability into science.
QUANTUM THEORY
QUANTUM THEORY
theory, the foundations of which were laid in 1900 by physicist Max Planck. According to this theory, atoms always emit or receive radiation energy only in portions, discontinuously, namely in certain quanta (energy quanta), the amount of energy of which is equal to the oscillation frequency (the speed of light divided by the wavelength) of the corresponding type of radiation, multiplied by the Planck action (see . Constant, Microphysics, and Quantum mechanics). Quantum theory was laid (by Einstein) as the basis of the quantum theory of light (corpuscular theory of light), according to which light also consists of quanta moving at the speed of light (light quanta, photons).
Philosophical Encyclopedic Dictionary. 2010 .
See what "QUANTUM THEORY" is in other dictionaries:
It has the following subsections (the list is incomplete): Quantum mechanics Algebraic quantum theory Quantum field theory Quantum electrodynamics Quantum chromodynamics Quantum thermodynamics Quantum gravity Superstring theory See also... ... Wikipedia
QUANTUM THEORY, a theory that, combined with the theory of RELATIVITY, formed the basis for the development of physics throughout the 20th century. It describes the relationship between MATTER and ENERGY at the level of ELEMENTARY or subatomic PARTICLES, as well as... ... Scientific and technical encyclopedic dictionary
quantum theory- Another way of research is studying the interaction of matter and radiation. The term “quantum” is associated with the name of M. Planck (1858 1947). This is the "black body" problem (an abstract mathematical concept for an object that accumulates all the energy... Western philosophy from its origins to the present day
Unites quantum mechanics, quantum statistics and quantum field theory... Big Encyclopedic Dictionary
Combines quantum mechanics, quantum statistics and quantum field theory. * * * QUANTUM THEORY QUANTUM THEORY combines quantum mechanics (see QUANTUM MECHANICS), quantum statistics (see QUANTUM STATISTICS) and quantum field theory... ... encyclopedic Dictionary
quantum theory- kvantinė teorija statusas T sritis fizika atitikmenys: engl. quantum theory vok. Quantentheorie, f rus. quantum theory, f pranc. théorie des quanta, f; théorie quantique, f … Fizikos terminų žodynas
Phys. a theory that combines quantum mechanics, quantum statistics, and quantum field theory. It is all based on the idea of a discrete (discontinuous) structure of radiation. According to quantum theory, any atomic system can be located in certain... ... Natural science. encyclopedic Dictionary
Quantum field theory is a quantum theory of systems with an infinite number of degrees of freedom (physical fields (See Physical fields)). Qt.p., which arose as a generalization of quantum mechanics (See Quantum mechanics) in connection with the problem of description... ... Great Soviet Encyclopedia
- (QFT), relativistic quantum. theory of physics systems with an infinite number of degrees of freedom. An example of such an electrical system. mag. field, for a complete description of which at any moment in time it is necessary to set the electrical intensities. and mag. fields at each point... Physical encyclopedia
QUANTUM FIELD THEORY. Contents:1. Quantum fields.................. 3002. Free fields and wave-particle duality.................... 3013. Interaction fields.........3024. Perturbation theory............... 3035. Divergences and... ... Physical encyclopedia
Books
- Quantum theory
- Quantum theory, Bohm D.. The book systematically presents non-relativistic quantum mechanics. The author analyzes in detail the physical content and examines in detail the mathematical apparatus of one of the most important...
- Quantum field theory Emergence and development Acquaintance with one of the most mathematized and abstract physical theories Issue 124, Grigoriev V. Quantum theory is the most general and deepest of the physical theories of our time. About how physical ideas about matter changed, how quantum mechanics arose, and then quantum mechanics...
I do not advise anyone who is interested in this issue to consult Wikipedia material.
What good things will we read there? Wikipedia notes that “quantum field theory” is “a branch of physics that studies the behavior of quantum systems with an infinitely large number of degrees of freedom - quantum (or quantized) fields; is the theoretical basis for the description of microparticles, their interactions and transformations.”
1. Quantum field theory: The first deception. Studying is, whatever you say, receiving and assimilating information that has already been collected by other scientists. Perhaps they meant "research"?
2. Quantum field theory: The second deception. There is not and cannot be an infinitely large number of degrees of freedom in any theoretical example of this theory. The transition from a finite number of degrees of freedom to an infinite number should be accompanied by not only quantitative, but also qualitative examples. Scientists often make generalizations of the following form: “Consider N = 2, after which we can easily generalize to N = infinity.” Moreover, as a rule, if the author has solved (or almost solved) the problem for N=2, it seems to him that he has accomplished the most difficult thing.
3. Quantum field theory: The third deception. “Quantum field” and “quantized field” are two big differences. Like between a beautiful woman and an embellished woman.
4. Quantum field theory: The fourth deception. About the transformation of microparticles. Another theoretical mistake.
5. Quantum field theory: The fifth deception. Particle physics as such is not science, but shamanism.
Read on.
“Quantum field theory is the only experimentally verified theory capable of describing and predicting the behavior of elementary particles at high energies (that is, at energies significantly higher than their rest energy).”
6. Quantum field theory: The sixth deception. Quantum field theory has not been confirmed experimentally.
7. Quantum field theory: The seventh deception. There are theories that are more consistent with experimental data, and in relation to them we can just as “reasonably” say that they are confirmed by experimental data. Consequently, quantum field theory is not the “only” of the “confirmed” theories.
8. Quantum field theory: The eighth deception. Quantum field theory is not capable of predicting anything. Not a single real experimental result can even be “confirmed” “after the fact” by this theory, let alone that anything could be calculated a priori with its help. Modern theoretical physics at the present stage makes all “predictions” on the basis of well-known tables, spectra and similar factual materials, which have not yet been “stitched” in any way by any of the officially accepted and recognized theories.
9. Quantum field theory: The ninth deception. At energies significantly higher than the rest energy, quantum theory not only gives nothing, but the formulation of the problem at such energies is impossible in the modern state of physics. The fact is that quantum field theory, like non-quantum field theory, like any of the currently accepted theories, cannot answer simple questions: “What is the maximum speed of the electron?” , as well as to the question “Is it equal to the maximum speed of any other particle?”
Einstein's theory of relativity states that the maximum speed of any particle is equal to the speed of light in a vacuum, that is, this speed cannot be achieved. But in this case, the question is valid: “What speed CAN be achieved?”
No answer. Because the statement of the Theory of Relativity is not true, and it was obtained from incorrect premises, incorrect mathematical calculations based on erroneous ideas about the admissibility of nonlinear transformations.
By the way, don't read Wikipedia at all. Never. My advice to you.
ANSWER TO THE PYROTECHNICIAN
In this particular context, I wrote that the description of QUANTUM FIELD THEORY IN WIKIPEDIA IS A DECEPTION.
My conclusion from the article: “Don’t read Wikipedia. Never. My advice to you."
How did you conclude that I “don’t like scientists” based on my denial of the scientific nature of some Wikipedia articles?
By the way, I never claimed that “Quantum field theory is a hoax.”
Exactly the opposite. Quantum field theory is an experimentally based theory, which is naturally not as meaningless as Special or General Relativity.
BUT STILL - quantum theory is ERRORAL IN PART OF POSTULATING those phenomena that CAN BE DERIVED AS CONSEQUENCES.
The quantum (quantized - more precisely and correctly) nature of the radiation of hot bodies is determined not by the quantum nature of the field as such, but by the discrete nature of the generation of oscillatory pulses, that is, the COUNTABLE NUMBER OF ELECTRON TRANSITIONS from one orbit to another - on the one hand, and the FIXED DIFFERENCE IN THE ENERGY of different orbits.
The fixed difference is determined by the properties of the movements of electrons in atoms and molecules.
These properties should be studied using the mathematical apparatus of closed dynamic systems.
I did it.
See articles at the end.
I have shown that the STABILITY OF ELECTRON ORBITS can be explained from ordinary electrodynamics, taking into account the limited speed of the electromagnetic field. From the same conditions, one can theoretically predict the geometric dimensions of the hydrogen atom.
The maximum outer diameter of a hydrogen atom is defined as twice the radius, and the radius corresponds to the potential energy of the electron, which is equal to the kinetic energy calculated from the relation E=mc^2/2 (em-ce-squared-in-half).
1. Bugrov S.V., Zhmud V.A. Modeling of nonlinear motions in dynamic problems of physics // Collection of scientific works of NSTU. Novosibirsk 2009. 1(55). pp. 121 – 126.
2. Zhmud V.A., Bugrov S.V. The modeling of the electron movements inside the atom on the base of the non-quantum physics. // Proceedings of the 18th IASTED International Conference “Applied Simulation and Modeling” (ASM 2009). Sept. 7-9, 2009. Palma de Mallorka, Spain. P.17 – 23.
3. Zhmud V.A. Justification of the non-relativistic non-quantum approach to modeling the motion of an electron in a hydrogen atom // Collection of scientific papers of NSTU. Novosibirsk 2009. 3(57). pp. 141 – 156.
By the way, among the possible answers to the question “Why do you dislike scientists so much?”
BECAUSE I LOVE SCIENCE.
Jokes aside: Scientists should not strive for love or non-love. They must strive for the truth. I “love with my mind” those who strive for truth, regardless of whether they are scientists or not. That is, I APPROVED. This is not why I love with my heart. Not for the pursuit of truth. Einstein strove for truth, but not always, not everywhere. As soon as he chose to strive to prove the infallibility of his theory, he completely forgot about the truth. After that, as a scientist, he faded quite considerably in my eyes. He should have thought more deeply about the gaseous nature of gravitational lenses, about the “postal” nature of information delay - we don’t judge the time of their departure by the arrival dates on letters! These two dates are always different. We don't identify them. Why, then, should one identify perceived time, perceived speed, etc., with real time, speed, etc.?
About the fact that I don't like readers? Hello! I'm trying to open their eyes. Is this not to love?
I even love the reviewers who object. Moreover, I especially love those who object reasonably. Those who seek not to object, but simply to deny, to assert the opposite without any reason, without reading into my arguments - I simply feel sorry for them.
“Why are they writing a note on something they haven’t even read?” - I think.
In conclusion, a joke for my readers who are tired of long discussions.
HOW TO WRITE A NOBEL SPEECH
1. Win a Nobel Prize.
2. Look around you. You will find many volunteer, unpaid helpers who would be honored to write this speech for you.
3. Read the four options given. Have a good laugh. Write anything - it will still be better than any of these options, and they, these options, are certainly better than what you can write bypassing point 1 of this sequence.
And most importantly, we refuse to notice that they are applicable only in some routine situations and for explaining the structure of the Universe they turn out to be simply incorrect.
Although something similar was expressed centuries ago by Eastern philosophers and mystics, Einstein was the first to talk about it in Western science. It was a revolution that our consciousness did not accept. With condescension we repeat: “everything is relative,” “time and space are one,” always keeping in mind that this is an assumption, a scientific abstraction that has little in common with our usual stable reality. In fact, it is precisely our ideas that poorly correlate with reality - amazing and incredible.
After the structure of the atom was discovered in general terms and its “planetary” model was proposed, scientists were faced with many paradoxes, to explain which a whole branch of physics appeared - quantum mechanics. It developed rapidly and made great progress in explaining the Universe. But these explanations are so difficult to understand that until now few people can understand them at least in general terms.
Indeed, most of the achievements of quantum mechanics are accompanied by such a complex mathematical apparatus that it simply cannot be translated into any human language. Mathematics, like music, is an extremely abstract subject, and scientists are still struggling to adequately express the meaning of, for example, the convolution of functions or multidimensional Fourier series. The language of mathematics is strict, but has little relation to our immediate perception.
Moreover, Einstein showed mathematically that our concepts of time and space are illusory. In reality, space and time are inseparable and form a single four-dimensional continuum. It is hardly possible to imagine it, because we are accustomed to dealing only with three dimensions.
Planetary theory. Wave or particle
Until the end of the 19th century, atoms were considered indivisible “elements.” The discovery of radiation allowed Rutherford to penetrate under the “shell” of the atom and formulate a planetary theory of its structure: the bulk of the atom is concentrated in the nucleus. The positive charge of the nucleus is compensated by negatively charged electrons, the sizes of which are so small that their mass can be neglected. Electrons revolve around the nucleus in orbits similar to the rotation of planets around the Sun. The theory is very beautiful, but a number of contradictions arise.
First, why don't negatively charged electrons "fall" onto the positive nucleus? Secondly, in nature, atoms collide millions of times per second, which does not harm them at all - how can we explain the amazing strength of the entire system? In the words of one of the “fathers” of quantum mechanics, Heisenberg, “no planetary system that obeys Newton’s laws of mechanics will ever return to its original state after a collision with another similar system.”
In addition, the dimensions of the nucleus, in which almost all the mass is collected, are extremely small compared to the whole atom. We can say that an atom is a void in which electrons rotate at breakneck speed. In this case, such an “empty” atom appears as a very solid particle. The explanation for this phenomenon goes beyond the classical understanding. In fact, at the subatomic level, the speed of a particle increases the more the space in which it moves is more limited. So the closer an electron is attracted to the nucleus, the faster it moves and the more it is repelled from it. The speed of movement is so high that “from the outside” the atom “looks solid”, just as the blades of a rotating fan look like a disk.
Data that do not fit well within the framework of the classical approach appeared long before Einstein. For the first time such a “duel” took place between Newton and Huygens, who tried to explain the properties of light. Newton argued that it was a stream of particles, Huygens considered light a wave. Within the framework of classical physics, it is impossible to reconcile their positions. After all, for her, a wave is a transmitted excitation of particles of the medium, a concept applicable only to many objects. None of the free particles can move along a wave-like trajectory. But an electron moves in a deep vacuum, and its movements are described by the laws of wave motion. What is excited here if there is no medium? Quantum physics offers a Solomonic solution: light is both a particle and a wave.
Probabilistic electron clouds. Nuclear structure and nuclear particles
Gradually it became more and more clear: the rotation of electrons in orbits around the nucleus of an atom is completely different from the rotation of planets around a star. Having a wave nature, electrons are described in terms of probability. We cannot say about an electron that it is located at such and such a point in space, we can only describe approximately in which areas it can be located and with what probability. Around the nucleus, electrons form “clouds” of such probabilities from the simplest spherical to very bizarre shapes, similar to photographs of ghosts.
But anyone who wants to finally understand the structure of the atom must turn to its basis, to the structure of the nucleus. The large elementary particles that make it up - positively charged protons and neutral neutrons - also have a quantum nature, which means they move the faster the smaller the volume they are contained in. Since the dimensions of the nucleus are extremely small even in comparison with an atom, these elementary particles rush around at quite decent speeds, close to the speed of light. For a final explanation of their structure and behavior, we will need to “cross” quantum theory with the theory of relativity. Unfortunately, such a theory has not yet been created and we will have to limit ourselves to a few generally accepted models.
The theory of relativity has shown (and experiments have proven) that mass is only one form of energy. Energy is a dynamic quantity associated with processes or work. Therefore, an elementary particle should be perceived as a probabilistic dynamic function, as interactions associated with the continuous transformation of energy. This gives an unexpected answer to the question of how elementary elementary particles are and whether they can be divided into “even simpler” blocks. If we accelerate two particles in an accelerator and then collide, we will get not two, but three particles, and completely identical ones. The third will simply arise from the energy of their collision - thus, they will separate and not separate at the same time!
Participant instead of observer
In a world where the concepts of empty space and isolated matter lose their meaning, a particle is described only through its interactions. In order to say something about it, we will have to “snatch” it from the initial interactions and, having prepared it, subject it to another interaction - measurement. So what are we measuring in the end? And how legitimate are our measurements in general if our intervention changes the interactions in which the particle participates - and therefore changes the particle itself?
In modern physics of elementary particles, more and more criticism is caused... by the very figure of the scientist-observer. It would be more appropriate to call him a “participant.”
An observer-participant is necessary not only to measure the properties of a subatomic particle, but also to determine these very properties, because they can only be discussed in the context of interaction with the observer. Once he chooses the method in which he will carry out measurements, and depending on this, the possible properties of the particle are realized. If you change the observing system, the properties of the observed object will also change.
This important moment reveals the deep unity of all things and phenomena. The particles themselves, continually changing into one another and into other forms of energy, do not have constant or precise characteristics - these characteristics depend on the way in which we choose to see them. If you need to measure one property of a particle, another will certainly change. Such a limitation is not associated with the imperfection of devices or other completely correctable things. This is a characteristic of reality. Try to accurately measure the position of a particle, and you will not be able to tell anything about the direction and speed of its movement - simply because it will not have them. Describe the exact motion of a particle - you will not find it in space. Thus, modern physics confronts us with problems of a completely metaphysical nature.
The principle of uncertainty. Place or impulse, energy or time
We have already said that we cannot talk about subatomic particles in the precise terms we are accustomed to; in the quantum world, we are left with only probability. This, of course, is not the probability that people talk about when betting on horse races, but a fundamental property of elementary particles. It’s not that they exist, but rather they can exist. It’s not that they have characteristics, but rather that they can have them. Scientifically speaking, a particle is a dynamic probabilistic circuit, and all its properties are in constant moving equilibrium, balancing like Yin and Yang in the ancient Chinese symbol of Taiji.
It is not for nothing that Nobel laureate Niels Bohr, elevated to the rank of nobility, chose this very sign and motto for his coat of arms: “Opposites complement each other.” Mathematically, the probability distribution represents uneven wave fluctuations. The greater the amplitude of a wave at a certain location, the higher the probability of a particle existing there. Moreover, its length is not constant - the distances between adjacent crests are not the same, and the higher the amplitude of the wave, the greater the difference between them. While amplitude corresponds to the particle's position in space, wavelength is related to the particle's momentum, that is, the direction and speed of its movement. The larger the amplitude (the more accurately the particle can be localized in space), the more uncertain the wavelength becomes (the less can be said about the particle's momentum). If we can determine the position of a particle with extreme precision, it will have no definite momentum at all.
This fundamental property is derived mathematically from the properties of waves and is called the uncertainty principle. The principle also applies to other characteristics of elementary particles. Another such interconnected pair is the energy and time of quantum processes. The faster the process, the more uncertain the amount of energy involved in it, and vice versa - energy can be accurately characterized only for a process of sufficient duration.
So, we understand: nothing definite can be said about a particle. It moves this way, or not there, or rather, neither here nor there. Its characteristics are this or that, or rather, not this or that. It is here, but it may be there, or it may not be anywhere. So does it even exist?