Can metal atoms form non-salt-forming oxides? Classification, preparation and properties of oxides. Chemical properties of oxides
Oxides complex substances are called, the molecules of which include oxygen atoms in the oxidation state - 2 and some other element.
can be obtained by direct interaction of oxygen with another element, and indirectly (for example, by decomposition of salts, bases, acids). Under normal conditions, oxides are in a solid, liquid and gaseous state, this type of compound is very common in nature. Oxides are found in the Earth's crust. Rust, sand, water, carbon dioxide are oxides.
They are salt-forming and non-salt-forming.
Salt-forming oxides- these are oxides that form salts as a result of chemical reactions. These are oxides of metals and non-metals, which, when interacting with water, form the corresponding acids, and when interacting with bases, they form the corresponding acidic and normal salts. For example, copper oxide (CuO) is a salt-forming oxide, because, for example, when it interacts with hydrochloric acid (HCl), a salt is formed:
CuO + 2HCl → CuCl 2 + H 2 O.
Other salts can be obtained as a result of chemical reactions:
CuO + SO 3 → CuSO 4.
Non-salt-forming oxides such oxides are called which do not form salts. An example is CO, N 2 O, NO.
Salt-forming oxides, in turn, are of 3 types: basic (from the word «
base »
), acidic and amphoteric.
Basic oxides such metal oxides are called, which correspond to hydroxides belonging to the class of bases. Basic oxides include, for example, Na 2 O, K 2 O, MgO, CaO, etc.
 Chemical properties of basic oxides
Chemical properties of basic oxides
1. Water-soluble basic oxides react with water to form bases:
Na 2 O + H 2 O → 2NaOH.
2. React with acidic oxides to form the corresponding salts
Na 2 O + SO 3 → Na 2 SO 4.
3. React with acids to form salt and water:
CuO + H 2 SO 4 → CuSO 4 + H 2 O.
4. React with amphoteric oxides:
Li 2 O + Al 2 O 3 → 2LiAlO 2.
If in the composition of the oxides as the second element there is a non-metal or a metal exhibiting the highest valence (usually from IV to VII), then such oxides will be acidic. Acidic oxides (acid anhydrides) are those oxides that correspond to hydroxides belonging to the class of acids. These are, for example, CO 2, SO 3, P 2 O 5, N 2 O 3, Cl 2 O 5, Mn 2 O 7, etc. Acidic oxides dissolve in water and alkalis to form salt and water.
Chemical properties of acidic oxides
1. Interact with water, forming acid:
SO 3 + H 2 O → H 2 SO 4.
But not all acidic oxides react directly with water (SiO 2, etc.).
2. React with base oxides to form salt:
CO 2 + CaO → CaCO 3
3. Interact with alkalis, forming salt and water:
CO 2 + Ba (OH) 2 → BaCO 3 + H 2 O.
Part amphoteric oxide includes an element that has amphoteric properties. Amphotericity is understood as the ability of compounds to exhibit acidic and basic properties, depending on the conditions. For example, zinc oxide ZnO can be both a base and an acid (Zn (OH) 2 and H 2 ZnO 2). Amphotericity is expressed in the fact that, depending on the conditions, amphoteric oxides exhibit either basic or acidic properties.
Chemical properties of amphoteric oxides
1. Interact with acids, forming salt and water:
ZnO + 2HCl → ZnCl 2 + H 2 O.
2. React with solid alkalis (when fusion), forming as a result of the reaction salt - sodium zincate and water:
ZnO + 2NaOH → Na 2 ZnO 2 + H 2 O.
When zinc oxide interacts with an alkali solution (the same NaOH), another reaction occurs:
ZnO + 2 NaOH + H 2 O => Na 2.
The coordination number is a characteristic that determines the number of the nearest particles: atoms or inov in a molecule or crystal. Each amphoteric metal has its own coordination number. For Be and Zn it is 4; For and, Al is 4 or 6; For and, Cr is 6 or (very rarely) 4;
Amphoteric oxides usually do not dissolve or react with water.
Still have questions? Want to know more about oxides?
To get help from a tutor - register.
The first lesson is free!
site, with full or partial copying of the material, a link to the source is required.

These are complex substances consisting of two chemical elements, one of which is oxygen with an oxidation state (-2). General formula of oxides: NSmOn, where m is the number of atoms of an element NS, a n- the number of oxygen atoms. Oxides can be solid (sand SiO 2, quartz varieties), liquid (hydrogen oxide H 2 O), gaseous (carbon oxides: carbon dioxide CO 2 and carbon monoxide gases).
The nomenclature of chemical compounds has evolved with the accumulation of factual material. At first, while the number of known compounds was small, it was widely used trivial names, not reflecting the composition, structure and properties of the substance, - red lead PL 3 O 4, litharge PLO, magnesia MgO, iron scale Fe 3 О 4, laughing gas N 2 O, white arsenic As 2 О 3 The trivial nomenclature was replaced by semi-systematic nomenclature - the name included indications of the number of oxygen atoms in the compound: nitrous oxide- for lower ones, oxide- for higher oxidation states; anhydride- for acidic oxides.
At present, the transition to the modern nomenclature is almost complete. According to international nomenclature, in the title oxide should indicate the valency of the element; for example, SO 2 - sulfur (IV) oxide, SO 3 - sulfur (VI) oxide, CrO - chromium (II) oxide, Cr 2 O 3 - chromium (III) oxide, CrO 3 - chromium (VI) oxide.

According to their chemical properties, oxides are divided into salt-forming and non-salt-forming.
 Types of oxides
Types of oxides Non-salt-forming such oxides are called which do not interact with either alkalis or acids and do not form salts. There are few of them, they include non-metals.
Salt-forming these are called oxides that react with acids or bases to form salt and water.
Among salt-forming oxides distinguish between oxides basic, acidic, amphoteric.
Basic oxides- these are the oxides to which the bases correspond. For example: CuO corresponds to the base Cu (OH) 2, Na 2 O - the base NaOH, Cu 2 O - CuOH, etc.

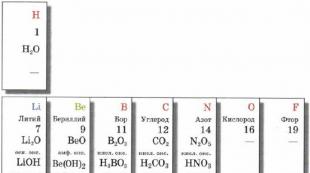
 Oxides in the periodic table
Oxides in the periodic table Typical reactions of basic oxides
1. Basic oxide + acid = salt + water (exchange reaction):
2. Basic oxide + acidic oxide = salt (compound reaction):
3. Basic oxide + water = alkali (compound reaction):

Acidic oxides are those oxides to which acids correspond. These are oxides of non-metals: N 2 O 5 corresponds to HNO 3, SO 3 - H 2 SO 4, CO 2 - H 2 CO 3, P 2 O 5 - H 4 PO 4 as well as metal oxides with a high value of oxidation states: Cr 2 + 6 O 3 corresponds to H 2 CrO 4, Mn 2 +7 O 7 - HMnO 4.
Typical reactions of acidic oxides
1. Acid oxide + base = salt + water (exchange reaction):
2. Acid oxide + basic oxide salt (compound reaction):
3. Acidic oxide + water = acid (compound reaction):

Such a reaction is possible, only if the acidic oxide is soluble in water.
Amphoteric called oxides, which, depending on the conditions, exhibit basic or acidic properties. These are ZnO, Al 2 O 3, Cr 2 O 3, V 2 O 5.
Amphoteric oxides do not combine directly with water.
Typical reactions of amphoteric oxides
1. Amphoteric oxide + acid = salt + water (exchange reaction):
2. Amphoteric oxide + base = salt + water or complex compound:

Basic oxides. TO the main include typical metal oxides, they correspond to hydroxides with the properties of bases.
Obtaining basic oxides
Oxidation of metals when heated in an oxygen atmosphere.
2Mg + O 2 = 2MgO
2Cu + O 2 = 2CuO
The method is not applicable for the preparation of alkali metal oxides. In reaction with oxygen, alkali metals usually give peroxides; therefore, the oxides Na 2 O, K 2 O are difficult to obtain.
Sulphide roasting
2CuS + 3O 2 = 2CuO + 2SO 2
4FeS 2 + 110 2 = 2Fe 2 O 3 + 8SO 2
The method is not applicable for sulphides of active metals, which are oxidized to sulphates.
Decomposition of hydroxides
Cu (OH) 2 = CuO + H 2 O
By thisthe method cannot be used to obtain oxides of alkali metals.
Decomposition of salts of oxygen-containing acids.
BaCO 3 = BaO + CO 2
2Pb (NO 3) 2 = 2PbO + 4N0 2 + O 2
4FeSO 4 = 2Fe 2 O 3 + 4SO 2 + O 2
Decomposition is easily carried out for nitrates and carbonates, including basic salts.
2 CO 3 = 2ZnO + CO 2 + H 2 O
Getting acidic oxides
Acidic oxides are represented by oxides of non-metals or transition metals in high oxidation states. They can be obtained by methods similar to those for the preparation of basic oxides, for example:
- 4P + 5O 2 = 2P 2 O 5
- 2ZnS + 3O 2 = 2ZnO + 2SO 2
- K 2 Cr 2 O 7 + H 2 SO 4 = 2CrO 3 ↓ + K 2 SO 4 + H 2 O
- Na 2 SiO 3 + 2HCl = 2NaCl + SiO 2 ↓ + H 2 O

Modern chemical science represents many different branches, and each of them, in addition to the theoretical basis, has great applied value, practical. Whatever you touch, everything around is chemical products. The main sections are inorganic and organic chemistry. Let us consider which main classes of substances are classified as inorganic and what properties they possess.
Main categories of inorganic compounds
These include the following:
- Oxides.
- Salt.
- Foundations.
- Acids.
Each of the classes is represented by a wide variety of compounds of inorganic nature and is important in almost any structure of human economic and industrial activity. All the main properties characteristic of these compounds, being in nature and receiving, are studied in the school chemistry course without fail, in grades 8-11.
There is a general table of oxides, salts, bases, acids, which presents examples of each of the substances and their state of aggregation, being in nature. Interactions describing chemical properties are also shown. However, we will look at each of the classes separately and in more detail.
Group of compounds - oxides
4. Reactions as a result of which the elements change CO
Me + n O + C = Me 0 + CO
1. Reagent water: acid formation (SiO 2 exclusion)
KO + water = acid
2. Reactions with bases:
CO 2 + 2CsOH = Cs 2 CO 3 + H 2 O
3. Reactions with basic oxides: salt formation
P 2 O 5 + 3MnO = Mn 3 (PO 3) 2
4. OVR reactions:
CO 2 + 2Ca = C + 2CaO,
They exhibit dual properties, interact according to the principle of the acid-base method (with acids, alkalis, basic oxides, acid oxides). They do not interact with water.
1.With acids: formation of salts and water
AO + acid = salt + H 2 O
2.With bases (alkalis): formation of hydroxo complexes
Al 2 O 3 + LiOH + water = Li
3. Reactions with acidic oxides: obtaining salts
FeO + SO 2 = FeSO 3
4. Reactions with RO: salt formation, fusion
MnO + Rb 2 O = double salt Rb 2 MnO 2
5. Fusion reactions with alkalis and alkali metal carbonates: salt formation
Al 2 O 3 + 2LiOH = 2LiAlO 2 + H 2 O
Each higher oxide, formed by both metal and non-metal, dissolves in water, gives a strong acid or alkali.
Organic and inorganic acids
In the classical sound (based on the positions of ED - electrolytic dissociation - acids are compounds that dissociate in an aqueous medium into H + cations and anions of acid residues An -. However, today acids have also been thoroughly studied in anhydrous conditions, therefore there are many different theories for hydroxides.
Empirical formulas of oxides, bases, acids, salts are composed only of symbols, elements and indices indicating their amount in a substance. For example, inorganic acids are expressed by the formula H + acidic residue n-. Organic matter has a different theoretical display. In addition to the empirical one, it is possible to write down a complete and abbreviated structural formula for them, which will reflect not only the composition and quantity of a molecule, but also the order of arrangement of atoms, their relationship with each other and the main functional group for carboxylic acids —COOH.
In an inorganic, all acids are divided into two groups:
- oxygen-free - HBr, HCN, HCL and others;
- oxygen-containing (oxo acids) - HClO 3 and everything where there is oxygen.
Also, inorganic acids are classified by stability (stable or stable - everything except carbonic and sulphurous, unstable or unstable - carbonic and sulphurous). In terms of strength, acids can be strong: sulfuric, hydrochloric, nitric, chloric and others, as well as weak: hydrogen sulfide, hypochlorous and others.

Organic chemistry offers far less variety. Acids that are organic in nature are carboxylic acids. Their common feature is the presence of the -COOH functional group. For example, HCOOH (formic), CH 3 COOH (acetic), C 17 H 35 COOH (stearic) and others.
There are a number of acids that are especially emphasized when considering this topic in a school chemistry course.
- Salt.
- Nitrogen.
- Orthophosphoric.
- Hydrobromic.
- Coal.
- Hydrogen iodide.
- Sulfuric.
- Acetic, or ethane.
- Butane, or oil.
- Benzoin.
These 10 acids in chemistry are the fundamental substances of the corresponding class both in the school course and in industry and synthesis in general.

Properties of inorganic acids
The main physical properties include, first of all, a different state of aggregation. After all, there are a number of acids in the form of crystals or powders (boric, orthophosphoric) under normal conditions. The overwhelming majority of the known inorganic acids are different liquids. Boiling and melting points also vary.
Acids can cause severe burns, as they have a force that destroys organic tissue and skin. Indicators are used to detect acids:
- methyl orange (in a normal environment - orange, in acids - red),
- litmus (in neutral - violet, in acids - red) or some others.
The most important chemical properties include the ability to interact with both simple and complex substances.
| What they interact with | Example reaction |
1. With simple substances, metals. A prerequisite: the metal must stand in the EHRNM before hydrogen, since the metals that are after hydrogen are not able to displace it from the composition of acids. The reaction always produces hydrogen gas and salt. | |
2. With bases. The result of the reaction is salt and water. Such reactions of strong acids with alkalis are called neutralization reactions. | Any acid (strong) + soluble base = salt and water |
| 3. With amphoteric hydroxides. Bottom line: salt and water. | 2HNO 2 + beryllium hydroxide = Be (NO 2) 2 (average salt) + 2H 2 O |
| 4. With basic oxides. Bottom line: water, salt. | 2HCL + FeO = iron (II) chloride + H 2 O |
| 5. With amphoteric oxides. The net effect is salt and water. | 2HI + ZnO = ZnI 2 + H 2 O |
6. With salts formed by weaker acids. The net effect is salt and weak acid. | 2HBr + MgCO 3 = magnesium bromide + H 2 O + CO 2 |
When interacting with metals, not all acids react in the same way. Chemistry (grade 9) at school involves a very shallow study of such reactions, however, even at this level, the specific properties of concentrated nitric and sulfuric acid are considered when interacting with metals.
Hydroxides: alkalis, amphoteric and insoluble bases
Oxides, salts, bases, acids - all these classes of substances have a common chemical nature, which is explained by the structure of the crystal lattice, as well as the mutual influence of atoms in the composition of molecules. However, while it was possible to give a very specific definition for oxides, it is more difficult to do it for acids and bases.
As well as acids, according to the theory of ED, bases are called substances that can decompose in an aqueous solution into metal cations Ме n + and anions of hydroxo groups ОН -.
- Soluble or alkalis (strong bases that change. Formed by metals of groups I, II. Example: KOH, NaOH, LiOH (that is, only elements of the main subgroups are taken into account);
- Slightly soluble or insoluble (medium strength, do not change the color of indicators). Example: hydroxide of magnesium, iron (II), (III) and others.
- Molecular (weak bases, in an aqueous medium reversibly dissociate into ions-molecules). Example: N 2 H 4, amines, ammonia.
- Amphoteric hydroxides (show dual basic acid properties). Example: beryllium, zinc, and so on.

Each group represented is studied in the school chemistry course in the "Foundations" section. Grade 8-9 chemistry involves a detailed study of alkalis and poorly soluble compounds.
The main characteristic properties of the bases
All alkalis and poorly soluble compounds are in nature in a solid crystalline state. At the same time, their melting points, as a rule, are low, and poorly soluble hydroxides decompose when heated. The color of the bases is different. If the alkali is white, then the crystals of poorly soluble and molecular bases can be of very different colors. The solubility of most compounds of this class can be seen in the table, which presents the formulas of oxides, bases, acids, salts, shows their solubility.
Alkalis can change the color of indicators as follows: phenolphthalein - raspberry, methyl orange - yellow. This is ensured by the free presence of hydroxyl groups in the solution. That is why poorly soluble bases do not give such a reaction.
The chemical properties of each group of bases are different.
| Chemical properties | ||
| Alkalis | Slightly soluble bases | Amphoteric hydroxides |
I. Interact with KO (total - salt and water): 2LiOH + SO 3 = Li 2 SO 4 + water II. Interact with acids (salt and water): normal neutralization reactions (see acids) III. Interact with AO to form a hydroxo complex of salt and water: 2NaOH + Me + n O = Na 2 Me + n O 2 + H 2 O, or Na 2 IV. Interact with amphoteric hydroxides to form hydroxocomplex salts: The same as with AO, only without water V. Interact with soluble salts to form insoluble hydroxides and salts: 3CsOH + iron (III) chloride = Fe (OH) 3 + 3CsCl Vi. React with zinc and aluminum in aqueous solution to form salts and hydrogen: 2RbOH + 2Al + water = complex with hydroxide ion 2Rb + 3H 2 | I. When heated, they can decompose: insoluble hydroxide = oxide + water II. Reactions with acids (total: salt and water): Fe (OH) 2 + 2HBr = FeBr 2 + water III. Interact with KO: Me + n (OH) n + KO = salt + H 2 O | I. React with acids to form salt and water: (II) + 2HBr = CuBr 2 + water II. Reacts with alkalis: total salt and water (condition: fusion) Zn (OH) 2 + 2CsOH = salt + 2H 2 O III. They react with strong hydroxides: the result is salts, if the reaction proceeds in an aqueous solution: Cr (OH) 3 + 3RbOH = Rb 3 |
These are most of the chemical properties that bases exhibit. The chemistry of bases is quite simple and obeys the general laws of all inorganic compounds.
Class of inorganic salts. Classification, physical properties
Based on the position of ED, salts can be called inorganic compounds, in an aqueous solution dissociating into metal cations Ме + n and anions of acid residues An n-. This is how salts can be imagined. The definition of chemistry gives more than one, but it is the most accurate.
Moreover, by their chemical nature, all salts are subdivided into:
- Acidic (containing a hydrogen cation). Example: NaHSO 4.
- Basic (containing a hydroxyl group). Example: MgOHNO 3, FeOHCL 2.
- Medium (consist only of a metal cation and an acid residue). Example: NaCL, CaSO 4.
- Double (includes two different metal cations). Example: NaAl (SO 4) 3.
- Complex (hydroxocomplexes, aquacomplexes and others). Example: K 2.
Salt formulas reflect their chemical nature, and also speak about the qualitative and quantitative composition of the molecule.

Oxides, salts, bases, acids have different solubility properties, which can be found in the corresponding table.
If we talk about the state of aggregation of salts, then one should notice their uniformity. They only exist in a solid, crystalline or powdery state. The range of colors is quite varied. Solutions of complex salts, as a rule, have bright, saturated colors.
Chemical interactions for the class of medium salts
They have similar chemical properties of base, acid, salt. Oxides, as we have already considered, differ somewhat from them in this factor.
In total, 4 main types of interactions can be distinguished for medium salts.
I. Interaction with acids (only strong in terms of ED) with the formation of another salt and a weak acid:
KCNS + HCL = KCL + HCNS
II. Reactions with soluble hydroxides with the appearance of salts and insoluble bases:
CuSO 4 + 2LiOH = 2LiSO 4 soluble salt + Cu (OH) 2 insoluble base
III. Interaction with other soluble salt to form insoluble salt and soluble:
PbCL 2 + Na 2 S = PbS + 2NaCL
IV. Reactions with metals standing in the EHRNM to the left of the one that forms the salt. In this case, the metal that reacts should not interact with water under normal conditions:
Mg + 2AgCL = MgCL 2 + 2Ag
These are the main types of interactions that occur with medium salts. The formulas of complex, basic, double and acidic salts speak for themselves about the specificity of the displayed chemical properties.

Formulas of oxides, bases, acids, salts reflect the chemical essence of all representatives of these classes of inorganic compounds, and in addition, give an idea of the name of the substance and its physical properties. Therefore, you should pay special attention to their writing. A huge variety of compounds is offered to us as a whole by an amazing science - chemistry. Oxides, bases, acids, salts are just a part of the immense variety.
General formula of oxides: E x O y
Oxygen has the second largest electronegativity value (after fluorine); therefore, most compounds of chemical elements with oxygen are oxides.
Salt-forming oxides include those oxides that are capable of interacting with acids or bases to form the corresponding salt and water. Salt-forming oxides include:
- basic oxides, which usually form metals with oxidation states +1, +2. React with acids, with acid oxides, with amphoteric oxides, with water (only oxides of alkali and alkaline earth metals). The basic oxide element becomes a cation in the resulting salt. Na₂O, CaO, MgO, CuO.
- acid oxides- oxides of non-metals, as well as metals in the oxidation state from +5 to +7. React with water, with alkalis, with basic oxides, with amphoteric oxides. The acidic oxide element is part of the anion of the resulting salt. Mn 2 O 7, CrO 3, SO 3, N 2 O 5.
- amphoteric oxides, which form metals with oxidation states from +3 to +5 (amphoteric oxides also include BeO, ZnO, PbO, SnO). React with acids, alkalis, acidic and basic oxides.
Non-salt-forming oxides do not interact with acids or bases, respectively, do not form. N 2 O, NO, CO, SiO.
According to the IUPAC nomenclature, the names of oxides are composed of the word oxide and the name of the second chemical element (with less electronegativity) in the genitive case:
Calcium oxide - CaO.
If an element is capable of forming several oxides, then their names should indicate the oxidation state of the element (in Roman numerals in brackets after the name):
Fe 2 O 3 - iron (III) oxide;
MnO 2 - manganese (IV) oxide.
It is allowed to use Latin prefixes to indicate the number of atoms of elements included in the oxide molecule:
Na 2 O — disodium oxide;
CO is carbon monoxide;
CO 2 - carbon dioxide.
The trivial names for some oxides are also often used:
Examples of solving problems on the topic "oxide formulas"
EXAMPLE 1
| Exercise | What is the mass of manganese (IV) oxide required to obtain 14.2 g of chlorine from hydrochloric acid? |
| Solution | Let's write the reaction equation: According to the reaction equation Let's find the amount of the substance: Calculate the mass of manganese (IV) oxide: |
| Answer | You need to take 17.4 g of manganese (IV) oxide. |
EXAMPLE 2
| Exercise | Upon oxidation of 16.74 g of a divalent metal, 21.54 g of oxide was formed. Determine the metal and calculate the equivalent masses of the metal and its oxide. |
| Solution | The mass of oxygen in the metal oxide is: |
Today we begin our acquaintance with the most important classes of inorganic compounds. Inorganic substances are divided according to their composition, as you already know, into simple and complex ones.
|
OXIDE |
ACID |
BASE |
SALT |
|
E x O y |
NnA A - acid residue |
Me (OH)b OH - hydroxyl group |
Me n A b |
Complex inorganic substances are divided into four classes: oxides, acids, bases, salts. We start with the oxide class.
OXIDES
Oxides
- these are complex substances, consisting of two chemical elements, one of which is oxygen, with a valency equal to 2. Only one chemical element - fluorine, combining with oxygen, forms not an oxide, but oxygen fluoride OF 2.
They are called simply - "oxide + element name" (see table). If the valence of a chemical element is variable, then it is indicated by a Roman numeral enclosed in parentheses after the name of the chemical element.
|
Formula |
Name |
Formula |
Name |
|
carbon monoxide (II) |
Fe 2 O 3 |
iron (III) oxide |
|
|
nitric oxide (II) |
CrO 3 |
chromium (VI) oxide |
|
|
Al 2 O 3 |
aluminium oxide |
zinc oxide |
|
|
N 2 O 5 |
nitric oxide (V) |
Mn 2 O 7 |
manganese (VII) oxide |
Classification of oxides
All oxides can be divided into two groups: salt-forming (basic, acidic, amphoteric) and non-salt-forming or indifferent.
|
Metal oxides Me x O y |
Nonmetal oxides notMe x O y |
|||
|
The main |
Acidic |
Amphoteric |
Acidic |
Indifferent |
|
I, II Me |
V-VII Me |
ZnO, BeO, Al 2 O 3, Fe 2 O 3, Cr 2 O 3 |
> II not me |
I, II not me CO, NO, N 2 O |
1). Basic oxides Are the oxides to which the bases correspond. Basic oxides include oxides metals 1 and 2 groups, as well metals side subgroups with valence I and II (except for ZnO - zinc oxide and BeO - beryllium oxide):

2). Acidic oxides Are oxides to which acids correspond. Acid oxides include nonmetal oxides (except for non-salt-forming - indifferent), as well as metal oxides side subgroups with a valency of V before Vii (For example, CrO 3 is chromium (VI) oxide, Mn 2 O 7 is manganese (VII) oxide):

3). Amphoteric oxides- these are oxides, which correspond to bases and acids. These include metal oxides major and minor subgroups with valence III , sometimes IV , as well as zinc and beryllium (For example, BeO, ZnO, Al 2 O 3, Cr 2 O 3).
4). Non-salt-forming oxides- these are oxides indifferent to acids and bases. These include nonmetal oxides with valence I and II (For example, N 2 O, NO, CO).
Conclusion: the nature of the properties of oxides primarily depends on the valence of the element.
For example, chromium oxides:
CrO (II- main);
Cr 2 O 3 (III- amphoteric);
CrO 3 (Vii- acidic).
Classification of oxides
(by solubility in water)
|
Acidic oxides |
Basic oxides |
Amphoteric oxides |
|
Soluble in water. Exception - SiO 2 (insoluble in water) |
Only oxides of alkali and alkaline earth metals dissolve in water (these are metals I "A" and II "A" groups, excluding Be, Mg) |
They do not interact with water. Insoluble in water |
Complete tasks:
1. Write down separately the chemical formulas of salt-forming acid and basic oxides.
NaOH, AlCl 3, K 2 O, H 2 SO 4, SO 3, P 2 O 5, HNO 3, CaO, CO.
2. Given substances : CaO, NaOH, CO 2, H 2 SO 3, CaCl 2, FeCl 3, Zn (OH) 2, N 2 O 5, Al 2 O 3, Ca (OH) 2, CO 2, N 2 O, FeO,
SO 3, Na 2 SO 4, ZnO, CaCO 3, Mn 2 O 7, CuO, KOH, CO, Fe (OH) 3
Obtaining oxides
Simulator "Interaction of oxygen with simple substances"
|
1. Combustion of substances (Oxidation with oxygen) |
a) simple substances Training apparatus |
2Mg + O 2 = 2MgO |
|
b) complex substances |
2H 2 S + 3O 2 = 2H 2 O + 2SO 2 |
|
|
2.Decomposition of complex substances (use acid table, see appendices) |
a) salts SALTt= BASIC OXIDE + ACID OXIDE |
СaCO 3 = CaO + CO 2 |
|
b) Insoluble bases Me (OH)bt= Me x O y+ H 2 O |
Cu (OH) 2 t = CuO + H 2 O |
|
|
c) oxygenated acids NnA =ACID OXIDE + H 2 O |
H 2 SO 3 = H 2 O + SO 2 |
Physical properties of oxides
At room temperature, most oxides are solids (CaO, Fe 2 O 3, etc.), some are liquids (H 2 O, Cl 2 O 7, etc.) and gases (NO, SO 2, etc.).



Chemical properties of oxides
|
CHEMICAL PROPERTIES OF BASIC OXIDES 1. Basic oxide + Acidic oxide = Salt (p. Compound) CaO + SO 2 = CaSO 3 2. Basic oxide + Acid = Salt + H 2 O (p. Exchange) 3 K 2 O + 2 H 3 PO 4 = 2 K 3 PO 4 + 3 H 2 O 3. Basic oxide + Water = Alkali (p. Compound) Na 2 O + H 2 O = 2 NaOH |
|
CHEMICAL PROPERTIES OF ACID OXIDES 1. Acid oxide + Water = Acid (p. Compound) C O 2 + H 2 O = H 2 CO 3, SiO 2 - does not react 2. Acid oxide + Base = Salt + H 2 O (p. Exchange) P 2 O 5 + 6 KOH = 2 K 3 PO 4 + 3 H 2 O 3. Basic oxide + Acidic oxide = Salt (p. Compound) CaO + SO 2 = CaSO 3 4. The less volatile displace the more volatile ones from their salts CaCO 3 + SiO 2 = CaSiO 3 + CO 2 |
|
CHEMICAL PROPERTIES OF AMPHOTHERIC OXIDES They interact with both acids and alkalis. ZnO + 2 HCl = ZnCl 2 + H 2 O ZnO + 2 NaOH + H 2 O = Na 2 [Zn (OH) 4] (in solution) ZnO + 2 NaOH = Na 2 ZnO 2 + H 2 O (when fusion) |
Application of oxides
Some oxides do not dissolve in water, but many enter into a compound reaction with water:
SO 3 + H 2 O = H 2 SO 4
CaO + H 2 O = Ca( OH) 2
The result is often highly desirable and useful compounds. For example, H 2 SO 4 is sulfuric acid, Ca (OH) 2 is slaked lime, etc.
If oxides are insoluble in water, then people skillfully use this property as well. For example, zinc oxide ZnO is a white substance, therefore it is used to prepare white oil paint (zinc white). Since ZnO is practically insoluble in water, zinc white can be used to paint any surfaces, including those that are exposed to atmospheric precipitation. Insolubility and non-toxicity make it possible to use this oxide in the manufacture of cosmetic creams and powders. Pharmacists make it an astringent and drying powder for external use.
Titanium (IV) oxide - TiO 2 possesses the same valuable properties. It also has a beautiful white color and is used to make titanium white. TiO 2 does not dissolve not only in water, but also in acids; therefore, coatings made of this oxide are especially resistant. This oxide is added to the plastic to give it a white color. It is part of enamels for metal and ceramic dishes.
Chromium (III) oxide - Cr 2 O 3 - very strong crystals of dark green color, insoluble in water. Cr 2 O 3 is used as a pigment (paint) in the manufacture of decorative green glass and ceramics. The GOI paste known to many (abbreviated from the name "State Optical Institute") is used for grinding and polishing optics, metal products, in jewelry.
Due to the insolubility and strength of chromium (III) oxide, it is also used in printing inks (for example, for coloring banknotes). In general, oxides of many metals are used as pigments for a wide variety of paints, although this is far from their only application.
Tasks for consolidation
1. Write down separately the chemical formulas of salt-forming acid and basic oxides.
NaOH, AlCl 3, K 2 O, H 2 SO 4, SO 3, P 2 O 5, HNO 3, CaO, CO.
2. Given substances : CaO, NaOH, CO 2, H 2 SO 3, CaCl 2, FeCl 3, Zn (OH) 2, N 2 O 5, Al 2 O 3, Ca (OH) 2, CO 2, N 2 O, FeO, SO 3, Na 2 SO 4, ZnO, CaCO 3, Mn 2 O 7, CuO, KOH, CO, Fe (OH) 3
Choose from the list: basic oxides, acidic oxides, indifferent oxides, amphoteric oxides and give them names.
3. Finish CCM, indicate the type of reaction, name the reaction products
Na 2 O + H 2 O =
N 2 O 5 + H 2 O =
CaO + HNO 3 =
NaOH + P 2 O 5 =
K 2 O + CO 2 =
Cu (OH) 2 =? +?
4. Carry out the transformations according to the scheme:
1) K → K 2 O → KOH → K 2 SO 4
2) S → SO 2 → H 2 SO 3 → Na 2 SO 3
3) P → P 2 O 5 → H 3 PO 4 → K 3 PO 4