How the external energy level is determined. External energy levels: structural features and their role in interactions between atoms. Self-help assignments
Answer from Ksenia Gareeva[guru]
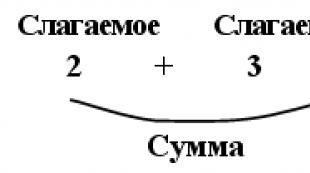
period number
Answer from Slava mikailov[newbie]
Answer from Argue[guru]
Energy level
From Wikipedia, the free encyclopedia
Energy level - the possible values of the energy of quantum systems, i.e. systems consisting of microparticles (electrons, protons and other elementary particles, atomic nuclei, atoms, molecules, etc.) and obeying the laws of quantum mechanics. It characterizes a certain state of a microparticle. Distinguish between electronic and intranuclear energy levels.
[edit]
Electronic energy levels
The modern concept of the orbital model of the atom, in which electrons move from one energy level to another, and the difference between the energy levels determines the size of the released or absorbed quantum. In this case, electrons cannot be in the intervals between energy levels. These gaps are called the energy gap.
An example is an electron in the orbital model of an atom - depending on the values of the principal quantum number n and the orbital quantum number l, the level of energy that the electron possesses changes. Accordingly, each pair of values of the numbers n and l corresponds to a certain energy level.
[edit]
Intranuclear energy levels
The term originated from research into radioactivity. Radiation radiation is divided into three parts: alpha rays, beta rays and gamma rays. Research has shown that alpha radiation is made up of helium atoms, beta radiation is a stream of fast moving electrons, and research on gamma rays has shown that the energy of electronic levels is not enough to create them. It became clear that the source of radioactive radiation (gamma rays) must be sought inside the atomic nucleus, that is, there are intranuclear energy levels, the energy of which is converted into photons of gamma radiation. Gamma rays have broadened the spectrum of known electromagnetic waves, and all waves shorter than 0.01 nm are gamma rays.
Try to imagine how small the atoms are compared to the size of the molecules themselves in this example.
Let's fill rubber ball gas. If we assume that a million molecules per second will emerge from the ball through a thin puncture, then it will take 30 billion years for all the molecules to escape from the ball. But the composition of one molecule can include two, three, and maybe several tens or even several thousand atoms!
Modern technology has made it possible to photograph both the molecule and the atom using a special microscope. The molecule was photographed at 70 million times magnification, and the atom - 260 million times.
For a long time, scientists believed that the atom was indivisible. Even a word atom translated from Greek means "indivisible". However, many years of research have shown that, despite their small size, atoms consist of even smaller parts ( elementary particles).
Isn't it true, the structure of the atom resembles Solar system ?
V the center of the atom - the nucleus around which electrons move at some distance
Core- the heaviest part of the atom, the mass of the atom is concentrated in it.
The nucleus and electrons have electric charges that are opposite in sign, but equal in magnitude.
The nucleus has a positive charge, the electrons are negative, so the atom as a whole is not charged.
Remember
All atoms have a nucleus and electrons. Atoms differ from each other: the mass and charge of the nucleus; the number of electrons.
Exercise
Count the number of electrons in the atoms of aluminum, carbon, hydrogen. Fill in the table.
|
· Atom name |
Number of electrons in an atom |
|
Aluminum atom |
|
|
Carbon atom |
|
|
Hydrogen atom |
Do you want to know more about the structure of the atom? Then read on.
The charge of the atomic nucleus is determined by the ordinal number of the element.
for instance , the ordinal number of hydrogen is 1 (determined by the Periodic Table of Mendeleev), which means that the charge of the atomic nucleus is +1.
The ordinal number of silicon is 14 (determined by the Periodic Table of Mendeleev), which means that the charge of the nucleus of the silicon atom is +14.
For an atom to be electrically neutral, the number of positive and negative charges in the atom must be the same
(the sum will be zero).
The number of electrons (negatively charged particles) is equal to the charge of the nucleus (positively charged particles) and is equal to the ordinal number of the element.
A hydrogen atom has 1 electron, silicon has 14 electrons.
Electrons in an atom move along energy levels.
The number of energy levels in an atom is determined by the number of the period, in which the element is located (also determined by the Periodic Table of Mendeleev)
For example, hydrogen is an element of the first period, so it has
1 is an energy level, and silicon is an element of the third period, therefore 14 electrons are distributed over three energy levels. Oxygen and carbon are elements of the third period, so electrons move through three energy levels.
Exercise
1.What is the charge of the nucleus in atoms chemical elements shown in the figure?
2. How many energy levels are there in the aluminum atom?
1 (2 points). Distribution of electrons by energy levels in a potassium atom:A. 2e, 8e, 8e, 1e C. 2e, 8e,
18th, 8th, 1st
B. 2nd, 1st D. 2nd, 8th, 1st
2 (2 points). The number of electrons in the outer electron layer of an aluminum atom:
A. 1 B. 2 C. 3 D.4
3 (2 points). A simple substance with the most pronounced metallic properties:
A. Calcium B. Barium C. Strontium G. Radium
4 (2 points). View chemical bond in a simple substance - aluminum:
A. Ionic B. Covalent polar
B. Metallic D. Covalent non-polar
5 (2 points). The number of energy levels for elements of one subgroup from top to bottom:
A. Changes periodically. B. Does not change.
B. Increases. G. Decreases.
6 (2 points). The lithium atom is different from the lithium ion:
A. Nuclear charge. B. The number of electrons in the external energy level.
B. The number of protons. D. The number of neutrons.
7 (2 points.). Reacts least vigorously with water:
A. Barium. B. Magnesium.
B. Calcium. G. Strontium
8 (2 points). Does not interact with sulfuric acid solution:
A. Aluminum. B. Sodium
B. Magnesium. G. Copper
9 (2 points). Potassium hydroxide does not interact with the substance, the formula of which is:
A. Na2O B. AlCl3
B. P2O5 G. Zn (NO3) 2
10 (2 points). The row in which all substances react with iron:
A. HCl, CO2, CO
B. CO2, HCl, S
B. H2, O2, CaO
G. O2, CuSO4, H2SO4
11 (9 points). Suggest three ways to get sodium hydroxide. Confirm the answer with the reaction equations.
12 (6 points). Carry out a chain of chemical transformations, making up the reaction equations in molecular and ionic forms, name the reaction products:
FeCl2 → Fe (OH) 2 → FeSO4 → Fe (OH) 2
13 (6 points). How, using any reagents (substances) and zinc, to get its oxide, base, salt? Write the reaction equations in molecular form.
14 (4 points). Write the equation for the chemical reaction of the interaction of lithium with nitrogen. Determine the reducing agent and oxidizing agent in this reaction
determine by:
A. group number;
B. period number;
B. serial number.
4. Which of the characteristics of chemical elements does not change in the main subgroups:
And the radius of the atom;
B is the number of electrons at the outer level;
B. the number of energy levels.
5. General in the structure of atoms of elements with serial numbers 7 and 15:
A. the number of electrons at the outer level, B. the charge of the nucleus;
B. number of energy levels.
Establish a correspondence between the symbol of a chemical element (in a given order) and the number of electrons at the outer energy level of its atom.Corresponding to the correct answers, you will compose the name of the installation, which will allow mankind to understand the structure of the atom even deeper (9 letters).
Number e per Element Symbol
Energy
level Mg Si I F C Ba Sn Ca Br
2 c a p o l y s e m
4 a o v k a t d h z
7 w y l l n g o l r
1 (3 points). The distribution of electrons over energy levels in the sodium atomA. 2 ē, 1 ē B. 2 ē, 4 ē B. 2 ē, 8 ē, 1ē. G. 2 ē, 8 ē, 3ē.
2 (4 points) Period number in Periodic table DI Mendeleev, in which there are no chemical elements-metals: A. 1. B. 2. C. 3. D. 4.
3 (3 points). The type of chemical bond in a simple calcium substance:
A. Ionic. B. Covalent polar. B. Covalent non-polar. G. Metallic.
4 (3 points). A simple substance with the most pronounced metallic properties:
A. Aluminum. B. Silicon. B. Magnesium. G. Sodium.
5 (3 points). The radius of the atoms of the elements of the 2nd period with an increase in the nuclear charge from an alkali metal to a halogen: A. It changes periodically. B. Does not change. B. Increases. G. Decreases.
6 (3 points). A magnesium atom is different from a magnesium ion:
A. Nuclear charge. B. Particle charge. B. The number of protons. D. The number of neutrons.
7 (3 points). Reacts most vigorously with water:
A. Potassium. B. Lithium. B. Sodium. G. Rubidium.
8 (3 points). Does not interact with dilute sulfuric acid:
A. Aluminum. B. Barium. B. Iron. G. Mercury.
9 (3 points). Beryllium hydroxide does not interact with a substance, the formula of which is:
A. NaOH (p p). B. NaCl (p_p). B. HC1 (p_p). G. H2SO4.
10 (3 points). The row in which all substances react with calcium:
A. CO2, H2, HC1. B. NaOH, H2O, HC1. B. C12, H2O, H2SO4. G. S, H2SO4, SO3.
PART B. Tasks with a free answer
11 (9 points). Suggest three ways to obtain iron (II) sulfate. Confirm the answer with the reaction equations.
12 (6 points). Determine the substances X, Y, Z, write down their chemical formulas.
Fe (OH) 3 (t) = X (+ HCl) = Y (+ NaOH) = Z (t) Fe2O3
13 (6 points). How, using any reagents (substances) and aluminum, to obtain an oxide, amphoteric hydroxide? Write the reaction equations in molecular form.
14 (4 points). Arrange the metals: copper, gold, aluminum, lead in order of increasing density.
15 (5 points). Calculate the mass of the metal obtained from 160 g of copper (II) oxide.
Rice. 7. Image of shapes and orientations
s-,p-,d-, orbitals using boundary surfaces.
Quantum numberm l are called magnetic ... It determines the spatial location of the atomic orbital and takes integer values from - l to + l through zero, that is, 2 l+ 1 values (Table 27).
Orbitals of one sublevel ( l= const) have the same energy. This state is called energy degenerate... So p-orbital - three times, d- five times, and f- sevenfold degenerate. Boundary surfaces s-,p-,d-, orbitals are shown in Fig. 7.
s -orbitals spherically symmetric for any n and differ from each other only in the size of the sphere. Their maximally symmetrical shape is due to the fact that at l= 0 and μ l = 0.
Table 27
Number of orbitals on energy sublevels
|
Orbital quantum number |
Magnetic quantum number |
The number of orbitals with a given value l |
|
m l | ||
|
–2, –1, 0, +1, +2 | ||
|
–3, –2, –1, 0, +1, +2, +3 |
p -orbitals exist at n≥ 2 and l= 1, therefore, three options for orientation in space are possible: m l= -1, 0, +1. All p-orbitals have a nodal plane dividing the orbital into two regions, so the boundary surfaces have the shape of dumbbells oriented in space at an angle of 90 ° relative to each other. The axes of symmetry for them are the coordinate axes, which are denoted p x , p y , p z .
d -orbitals are determined by the quantum number l = 2 (n≥ 3), at which m l= –2, –1, 0, +1, +2, that is, they are characterized by five variants of orientation in space. d-orbitals oriented by the blades along the coordinate axes are designated d z² and d x ²– y², and the blades oriented along the bisectors of the coordinate angles - d xy , d yz , d xz .
Seven f -orbitals corresponding l = 3 (n≥ 4) are depicted as boundary surfaces.
Quantum numbers n, l and m do not fully characterize the state of an electron in an atom. It has been experimentally established that the electron has one more property - spin. Simplified, spin can be represented as the rotation of an electron around its own axis. Spin quantum number m s has only two meanings m s= ± 1/2, representing two projections of the angular momentum of the electron onto the selected axis. Electrons with different m s indicated by up and down arrows.
Sequence filling atomic orbitals
The population of atomic orbitals (AO) with electrons is carried out according to the principle of least energy, the Pauli principle, the Gund rule, and for many-electron atoms - the Klechkovsky rule.
Least Energy Principle requires electrons to populate an AO in order of increasing electron energy in these orbitals. This reflects the general rule - the maximum stability of the system corresponds to the minimum of its energy.
Principle Pauli (1925) prohibits electrons with the same set of quantum numbers in a many-electron atom. This means that any two electrons in an atom (or molecule, or ion) must differ from each other by the value of at least one quantum number, that is, in one orbital there can be no more than two electrons with different spins (paired electrons). Each sublevel contains 2 l+ 1 orbitals containing no more than 2 (2 l+ 1) electrons. Hence it follows that the capacity s-orbitals - 2, p-orbitals - 6, d-orbitals - 10 and f-orbitals - 14 electrons. If the number of electrons for a given l sum from 0 to n- 1, then we get the formula Bora -Bury, which determines the total number of electrons at a level with a given n:
This formula does not take into account the electron-electron interaction and ceases to be fulfilled at n ≥ 3.
Orbitals with the same energies (degenerate) are filled in accordance with rule Gunda : the lowest energy is possessed by the electron configuration with the maximum spin. This means that if there are three electrons on the p-orbital, then they are located like this:, and the total spin S= 3/2, not like this:, S=1/2.
Klechkovsky rule (principle of least energy). In many-electron atoms, as in the hydrogen atom, the state of the electron is determined by the values of the same four quantum numbers, but in this case the electron is not only in the field of the nucleus, but also in the field of other electrons. Therefore, the energy in many-electron atoms is determined not only by the main, but also by the orbital quantum number, or rather by their sum: the energy of atomic orbitals increases as the sumn + l; with the same amount, the level with the lower one is filled firstnand bigl. The energy of atomic orbitals increases according to the series:
|
1s<2s<2p<3s<3p<4s≈3d<4p<5s≈4d<5p<6s≈4f≈5d<6p<7s≈5f≈6d<7p. |
So, four quantum numbers describe the state of an electron in an atom and characterize the energy of the electron, its spin, the shape of the electron cloud and its orientation in space. When an atom passes from one state to another, the electron cloud is rearranged, that is, the values of the quantum numbers change, which is accompanied by the absorption or emission of energy quanta by the atom.
What happens to the atoms of elements during chemical reactions? What do the properties of elements depend on? One answer can be given to both of these questions: the reason lies in the structure of the external. In our article we will consider the electronic of metals and non-metals and find out the relationship between the structure of the external level and the properties of the elements.
Special properties of electrons
During the passage of a chemical reaction between the molecules of two or more reagents, changes occur in the structure of the electronic shells of atoms, while their nuclei remain unchanged. First, let's get acquainted with the characteristics of electrons located at the most distant levels of the atom from the nucleus. Negatively charged particles are arranged in layers at a certain distance from the nucleus and from each other. The space around the nucleus, where it is most possible to find electrons, is called the electron orbital. About 90% of the negatively charged electron cloud is condensed in it. The electron itself in an atom exhibits the property of duality; it can simultaneously behave both as a particle and as a wave.
Rules for filling the electron shell of an atom
The number of energy levels at which the particles are located is equal to the number of the period where the element is located. What does the electronic composition indicate? It turned out that at the external energy level for s- and p-elements of the main subgroups of small and large periods corresponds to the group number. For example, lithium atoms of the first group, which have two layers, have one electron on the outer shell. Sulfur atoms contain six electrons at the last energy level, since the element is located in the main subgroup of the sixth group, etc. If we are talking about d-elements, then the following rule exists for them: the number of external negative particles is 1 (for chromium and copper) or 2. This is explained by the fact that as the charge of the atomic nucleus increases, the inner d-sublevel is first filled and the outer energy levels remain unchanged.
Why do the properties of elements of small periods change?
Periods 1, 2, 3 and 7 are considered small. A smooth change in the properties of elements as nuclear charges increase, ranging from active metals to inert gases, is explained by a gradual increase in the number of electrons at the external level. The first elements in such periods are those whose atoms have only one or two electrons, which can easily be detached from the nucleus. In this case, a positively charged metal ion is formed.

Amphoteric elements, for example, aluminum or zinc, fill their external energy levels with a small number of electrons (1 for zinc, 3 for aluminum). Depending on the conditions of the chemical reaction, they can exhibit both the properties of metals and non-metals. Non-metallic elements of small periods contain from 4 to 7 negative particles on the outer shells of their atoms and complete it up to an octet, attracting the electrons of other atoms. For example, a non-metal with the highest electronegativity index - fluorine, has 7 electrons on the last layer and always takes one electron not only from metals, but also from active non-metallic elements: oxygen, chlorine, nitrogen. Small periods, as well as large ones, end with inert gases, whose monatomic molecules have completely completed external energy levels up to 8 electrons.
Features of the structure of atoms of long periods
Even rows of 4, 5, and 6 periods consist of elements, the outer shells of which contain only one or two electrons. As we said earlier, they fill the d- or f-sublevels of the penultimate layer with electrons. These are usually typical metals. Their physical and chemical properties change very slowly. Odd rows contain elements in which the external energy levels are filled with electrons according to the following scheme: metals - amphoteric element - non-metals - inert gas. We have already observed its manifestation in all small periods. For example, in the odd row of the 4th period, copper is a metal, zinc is amphoteric, then from gallium to bromine there is an increase in non-metallic properties. The period ends with krypton, whose atoms have a fully completed electron shell.

How to explain the division of elements into groups?
Each group - and there are eight of them in the short form of the table, is also divided into subgroups, called main and secondary. This classification reflects the different position of the electrons on the external energy level of the atoms of the elements. It turned out that in the elements of the main subgroups, for example, lithium, sodium, potassium, rubidium and cesium, the last electron is located at the s-sublevel. Elements of the 7th group of the main subgroup (halogens) fill their p-sublevel with negative particles.
For representatives of side subgroups, such as chromium, filling with electrons of the d-sublevel will be typical. And the elements of the family accumulate negative charges at the f-sublevel of the penultimate energy level. Moreover, the group number, as a rule, coincides with the number of electrons capable of forming chemical bonds.

In our article, we found out what structure the external energy levels of atoms of chemical elements have, and determined their role in interatomic interactions.